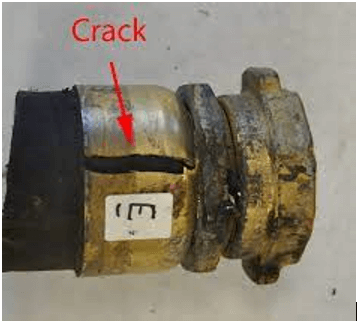
RESOURCES | post
Category: Water & Wastewater Treatment
Importance of amines for mitigating corrosion
Neutralizing amines are commonly used in many industrial applications to control corrosion. In particular, prominent applications include mitigating corrosion in refinery overhead systems [1-6] and in nuclear power plants [7-12]. In refining, various amines are added to neutralize hydrogen chloride, which results from the hydrolysis of alkaline earth chlorides that are found in crude oil. However, while the injection of amines reduces the risk of acid corrosion, it may lead to the formation of amine hydrochloride salts. Amine hydrochlorides may form either solid or concentrated aqueous phases, which may cause aggressive corrosion in refinery overheads. Thus, it is important to understand and to be able to predict the conditions that lead to such phenomena. Because of the inherent difficulties in monitoring the phase behavior in actual refinery settings, a thermodynamic model that can realistically predict these phenomena is of great value. Such a model is unavoidably complex because refinery overhead systems are multicomponent mixtures of hydrocarbons, water, acid gases, and amines.
A tremendous amount of research has been devoted in the literature to the thermodynamic behavior of amine systems that are used for CO2 capture and acid gas removal applications. Much less work has been published on the behavior of amines in the context of corrosion control in refining. Although there is a substantial overlap in the amine mixture properties that are required for acid gas removal and for corrosion control, the fundamental difference lies in the fact that refinery corrosion control is inextricably linked with the presence of hydrogen chloride, which induces the formation of amine hydrochlorides. Therefore, OLI has focused its modeling efforts on the properties of amine hydrochlorides as well as on those of the underlying amines.
The neutralizing amines that are used in the refining industry include primary alkyl amines (e.g., methylamine, ethylamine, propylamine, butylamine, cyclohexylamine, etc.), secondary alkyl amines (e.g., dimethylamine or diethylamine), tertiary alkyl amines (e.g., trimethylamine), alkoxy amines (e.g., 3-methoxypropylamine), cyclic ether amines (e.g., morpholine, N-methylmorpholine, N-ethylmorpholine, etc.), and alkanolamines (e.g., ethanolamine, diethanolamine, methyldiethanolamine, dimethylethanolamine, dimethylisopropanolamine, diglycolamine, etc.) Because of the differences in their molecular structure, these amines differ with respect to their volatility and their phase equilibria with water and hydrocarbons. Thus, their partitioning between the gas phase, hydrocarbon-rich liquid phase and aqueous phase differs widely. Also, the amines differ with respect to their propensity to form solid amine hydrochlorides or concentrated aqueous solutions containing amine hydrochlorides in an ionic form. Frequently, mixtures of amines are used, which additionally complicates the prediction of their behavior in refinery environments.
In order to predict the possibility of corrosion in refinery overheads, a thermodynamic model has been constructed for multicomponent mixtures that include water, amines, amine hydrochlorides, hydrogen chloride, carbon dioxide, and hydrocarbons. In general, such mixtures may form multiphase systems, including a gas phase, a hydrocarbon-rich liquid phase, an aqueous phase and solid amine hydrochloride phases. A conceptual scheme of the phases, species, and phase equilibria in such systems is shown in Fig. 1. In this figure, the amines are denoted as RNH2 for simplicity.
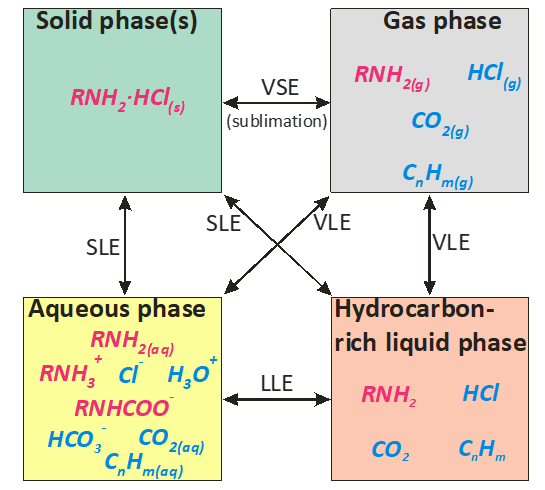
Fig. 1. A conceptual scheme of the phases, species, and phase equilibria that may appear in conjunction with the application of neutralizing amines.
In order to reproduce the behavior of neutralizing amines, the OLI model simultaneously reproduces:
- Vapor-liquid equilibria for mixtures of amines with water, which is necessary to predict the partitioning of amines between the vapor and aqueous phases;
- Vapor-solid equilibria for amine hydrochlorides, which determines the possibility of precipitation of amine hydrochlorides from the gas phase;
- Solid-liquid and solid-liquid-vapor equilibria for amine hydrochlorides in water, which influence the transition between solid amine hydrochlorides and concentrated amine hydrochloride solutions, the latter being of particular interest for predicting conditions that are conducive to corrosion;
- Speciation in the aqueous phase involving the equilibrium between molecular and ionized forms of the amines, dissociation of acid gases and formation of carbamate ions in the presence of CO2;
- Partitioning of amines and acid gases between the gas phase and the hydrocarbon-rich liquid phase, which influences the availability of the amines and HCl in the aqueous phase.
In order to develop a thermodynamic model that satisfies conditions (i-v), OLI’s Mixed-Solvent Electrolyte (MSE) computational framework has been applied (Wang et al. [13-15]). This framework is particularly suitable to capture the unique characteristics of amine hydrochlorides. On the one hand, amine hydrochlorides form strong electrolytes. On the other hand, they have relatively low melting points and are much more volatile than the majority of salts or ionic liquids. Thus, the model needs to reproduce vapor-solid as well as vapor-liquid and vapor-liquid-solid equilibria in systems containing amine hydrochlorides. Here, we show the results of modeling for selected mixtures containing water, amines and their corresponding hydrochlorides, HCl, and CO2.
Chemical reactions in amine systems
To model the behavior of both amines and amine hydrochlorides, the MSE model includes all important chemical reactions. A key reaction is the hydrolysis (or protonation) of the amine. For example, the hydrolysis reaction of methylamine is written as
CH3NH2(aq) + H20 = CH3NH3 ++ OH– (1)
To illustrate the hydrolysis of secondary and tertiary amines, we can use morpholine and its N-methyl and N-ethyl derivatives as examples:
O(CH2CH2)2NR(aq) + H2O = O(CH2CH2)2NRH+ + OH– (2)
where R represents H for morpholine, CH3 for N-methylmorpholine and C2H5 for N-ethylmorpholine. Further, primary and secondary amines undergo reactions with carbon dioxide that lead to the formation of carbamate ions. In the case of morpholine, the carbamate formation reaction is
O(CH2CH2)2NH(aq) + HCO3– = O(CH2CH2)2NCOO– + H2O (3)
These amine speciation reactions are considered in conjunction with the self-dissociation of water, i.e.,
2H2O = H3O+ + OH– (4)
and with the dissociation of dissolved acid gases, i.e., HCl and CO2:
HCI(aq) + H2O = H3O+ + CI– (5)
CO2(aq) + 2H2O = H3O+ + HCO3– (6)
HCO3– + H2O = H3O+ + CO23– (7)
Solid amine hydrochlorides may form as a result of gas-phase reactions between an amine and HCl. Thus, for methylamine hydrochloride, the solid-vapor equilibrium is defined as
CH3NH2HCI(s) = CH3NH2(g) + HCI(g) (8)
In the presence of a liquid phase, amine hydrochlorides may also undergo solid-liquid equilibria, i.e.,
CH3NH2HCI(s) = CH3NH3+ + CI– (9)
Phase equilibrium predictions
Amine – water systems.
Predicting vapor-liquid equilibria of mixtures containing amines and water is the key prerequisite for modeling the behavior of amines in refinery overheads. Figure 2 shows examples of such predictions for two representative amines. The predictions are in very good agreement with experimental data. The two amines shown in Fig. 2 strongly differ with respect to their volatility. Whereas methylamine is much more volatile than water, morpholine and its derivatives are less volatile than water. Accurately capturing the volatility of the amines is essential for the accuracy of the model.
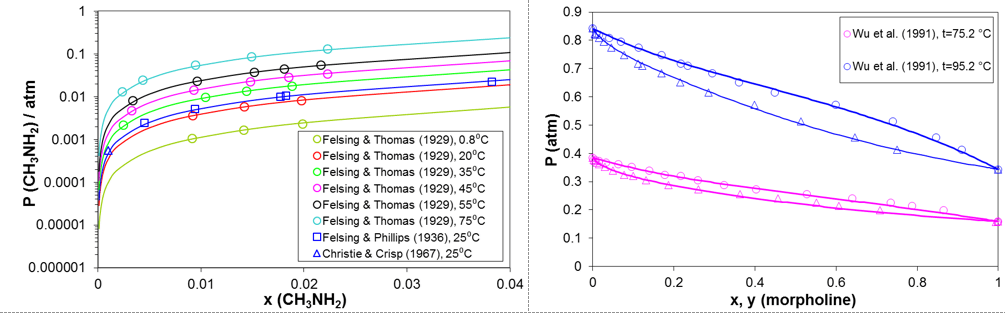
Figure 2. Prediction of vapor-liquid equilibria for mixtures of methylamine and morpholine with water using the MSE model.
As indicated by reactions (1) and (2), amines undergo hydrolysis reactions and are weak bases. It is the hydrolysis reaction that makes the amines effective as neutralizers. Figure 3 shows the prediction of the dissociation constants, Kb, for selected amines for which experimental data are available in the widest temperature ranges, i.e., for methylamine and morpholine. It should be noted that the maximum in the dissociation constant is a common feature of most amines although the location of the maximum differs depending on the structure of the amine.
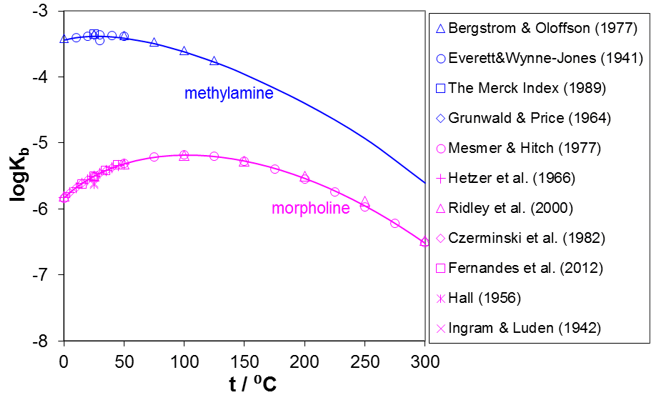
Figure 3. Prediction of dissociation constants for methylamine and morpholine in water
Pure amine hydrochlorides.
Unlike most pure components, amine hydrochlorides undergo a chemical reaction in conjunction with their sublimation or melting. This is due to the fact that amine hydrochlorides do not exist in a molecular form in the gas phase and decompose to the constituent amine and hydrochloride molecules. Figure 4 shows the equilibrium pressure for the reactive vapor-solid and vapor-liquid equilibria of pure morpholine and its substituted derivatives. The solid line indicates vapor-solid equilibrium whereas the dashed line depicts vapor-liquid equilibrium. The dashed vapor-liquid line is extended towards lower temperatures (i.e., within the metastable range of the liquid) to indicate the change in the slope of the two equilibrium curves at the triple point. The model accurately represents the available experimental data for both vapor-solid and vapor-liquid equilibria. The low equilibrium pressures in the temperature range below ca. 140 °C are particularly important because they are associated with the strong driving force for the formation of the solid hydrochloride in refinery overhead environments even when the partial pressures of amines and HCl in the gas phase are fairly low.
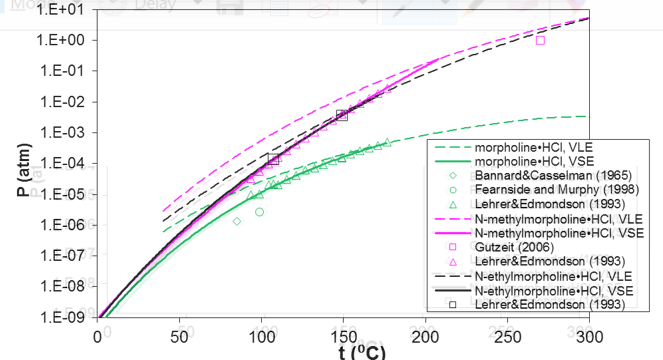
Figure 4. Prediction of vapor pressures of solid (solid lines) and liquid (dashed lines) hydrochlorides of morpholine, N-methylmorpholine, and N-ethylmorpholine. The dashed lines are extended into the metastable region of liquid amine hydrochlorides below their triple points.
Amine hydrochloride – water mixtures.
Simultaneous modeling of vapor-liquid and solid-liquid equilibria is essential for predicting the ionic dew point and salt point in refinery overheads. As an example, Figure 5 shows the solid-liquid equilibria in the methylamine hydrochloride – water system. The SLE curve in Figure 11 has two branches. The lower branch represents the solubility of ice whereas the upper branch is the solubility of solid methylamine hydrochloride. The upper branch terminates at the melting point of pure methylamine hydrochloride (226 °C). Figure 6 compares the calculated and experimental VLE results in this system for two isotherms (25 °C and 100 °C). The horizontal portion of the VLE curve starts at the solubility point at each temperature (consistent with the solubility in Fig. 5) and corresponds to three-phase solid-liquid-vapor equilibria, for which the equilibrium pressure is constant. The model represents both the SLE and VLE data in the complete concentration and temperature ranges within experimental uncertainty.
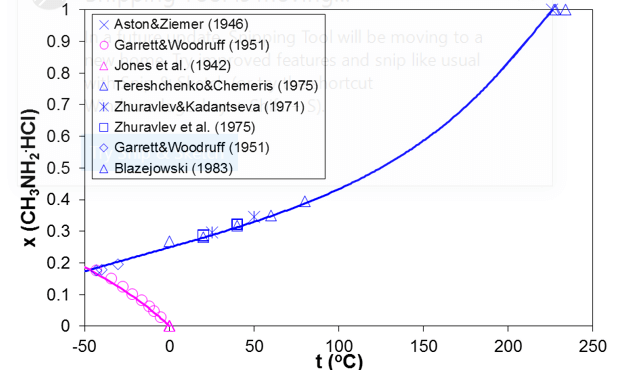
Figure 5. Prediction of solid solubilities for the mixture of methylamine hydrochloride with water
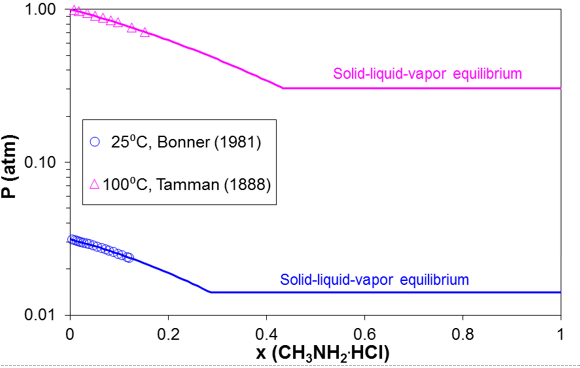
Figure 6. Prediction of vapor-liquid-solid equilibria for mixtures of methylamine hydrochloride with water
Amine – water – CO2 systems.
Carbon dioxide is ubiquitous in refinery applications and, despite being a much weaker acid than HCl, may affect the formation of amine hydrochlorides by contributing to the neutralization of amines and by forming carbamate ions. Thus, the effects of HCl and CO2 need to be considered simultaneously in the simulation of the performance of neutralizing amines. Figure 7 compares the calculated and experimental partial pressures of CO2 as a function of the CO2 loading (i.e., the CO2/morpholine molar ratio) at temperatures up to 120 °C. A corresponding speciation plot is shown on the right-hand side of Fig.7, in which the relative amounts of the species are indicated by the areas delimited by the lines. The model simultaneously reproduces the VLE and speciation data. As shown in Figure 7, the fraction of neutral amine, O(CH2CH2)2NH, rapidly diminishes with increasing CO2/morpholine ratio and becomes negligible as the ratio exceeds ca. 0.6. The fraction of the morpholinium ion, O(CH2CH2)2NH2+, and the morpholine carbamate ion, O(CH2CH2)2NCOO–, increase with the CO2/morpholine ratio and reach fairly constant values at the ratios above ca. 0.7. Thus, the significant concentration of the carbamate ion can be expected to interfere with the formation of amine hydrochloride salts.
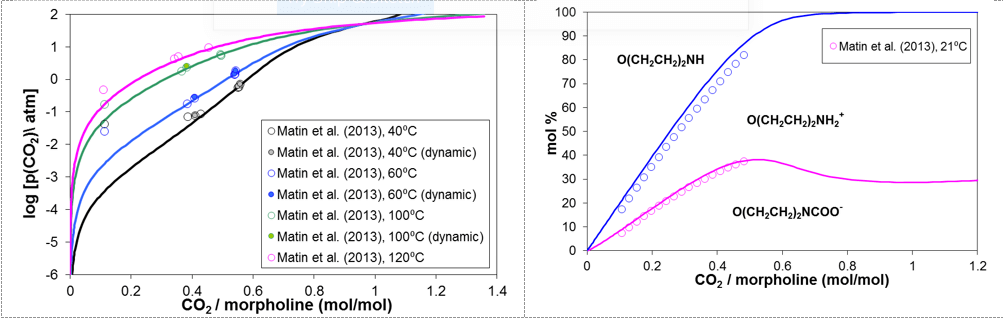
Figure 7. Prediction of vapor-liquid equilibria (left diagram) and speciation (right diagram) for the mixture of morpholine, water, and carbon dioxide.
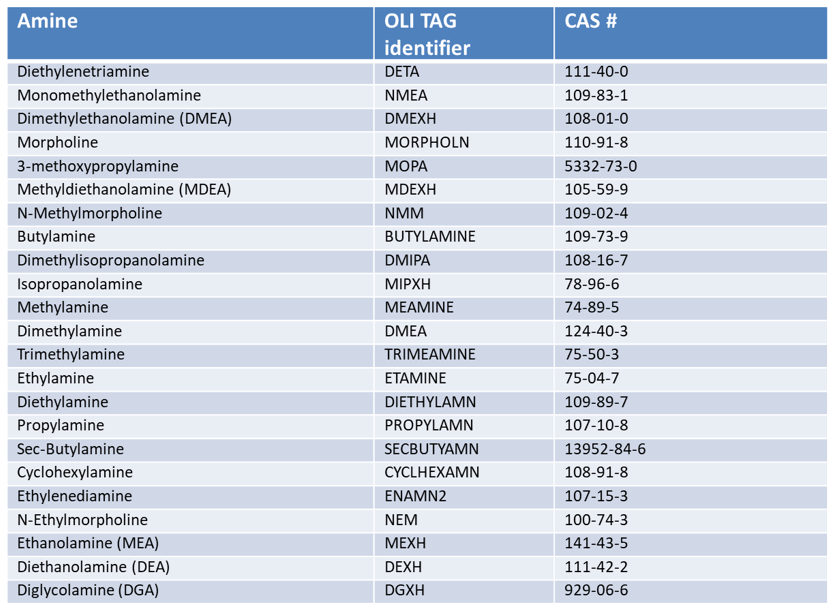
Table 1. Amines and corresponding amine hydrochlorides in the MSE database. Note that additional amines are included in the MSE model, but without the corresponding hydrochlorides.
Application of the model to refinery overhead modeling in the OLI software
As described by Patel et al. [5] and Armistead et al. [6], the combined model can be applied to predict the behavior of neutralizing amines in refinery overhead environments. Specifically, it has been used to study the evolution of the phase behavior of refinery streams as a function of temperature. The corrosive properties of streams can be analyzed by calculating three characteristic quantities, i.e., the salt point, ionic dew point, and water dew point [6]. The salt point is the temperature at which a solid amine hydrochloride precipitates from the gas phase and indicates the possibility of solid phase deposition downstream. The ionic dew point is the temperature that corresponds to the incipient formation of a typically small amount of a concentrated aqueous amine hydrochloride solution, which is highly corrosive. Finally, the water dew point is the temperature (or temperature range) at which the bulk of the water in the stream starts to condense, forming a dilute and typically non-corrosive aqueous solution. In general, it is desirable to minimize the temperature range between the ionic dew point and water dew point because the corrosive amine hydrochloride solution exists in this range. This can be achieved by modifying the stream conditions, implementing water washing, and optimizing the selection of amines. The MSE model, as implemented in the OLI software, provides a computational tool for this purpose. Table 1 shows the amines that are supported in the OLI Software platform v 11.5.
OLI Systems’ simulation technology, which is based on the MSE model, is available in OLI Studio V11.5, OLI Flowsheet ESP V11.5, OLI Cloud APIs and other OLI software products.
Contact OLI at https:/www.olisystems.com/contact-us for more information or to schedule a meeting with an OLI expert.
References
[1] D.P. Valenzuela, A.K. Dewan, Refinery crude column overhead corrosion control, amine neutralizer electrolyte thermodynamics, thermochemical properties and phase equilibria, Fluid Phase Equilibria, 158-160 (1999) 829-834.
[2] V.K. Braden, P.R. Petersen, Crude Unit Overhead Corrosion Control, CORROSION/1998, paper no. 585, NACE International (1998).
[3] R. Rechtien, G.G. Duggan, Identifying the Impacts of Amine Contamination on Crude Units, CORROSION/2006, paper no. 06581, NACE International (2006).
[4] J. Gutzeit, Crude Unit Corrosion Guide. A Complete How-To Manual, PCC, 2006.
[5] A. Patel, E. Vetters, A. Anderko, M. Lencka, Use of Ionic Modeling to Gain Insight on Crude Unit Overhead Corrosion, CORROSION /2012, paper no. C2012-0001209, NACE International (2012).
[6] K. Armistead, D. Leslie, R. Strong, Crude Unit Overhead Corrosion Control Successfully Driven by Ionic Modeling, CORROSION/2015, paper no. 6010, NACE International (2015).
[7] D.D. Macdonald, G.A. Cragnolino, Corrosion of Steam Cycle Materials, in: P. Cohen (Ed.) The ASME Handbook on Water Technology for Thermal Power Systems, American Society of Mechanical Engineers, New York, NY, 1989.
[8] P.V. Balakrishnan, Volatility of amines used for water treatment in steam generating systems, Can. J. Chem., 56 (1978) 2620-2623.
[9] D. Wesolowski, P. Bénézeth, D. Palmer, L. Anovitz, Protonation Constants of Morpholine, Dimethylamine and Ethanolamine to 290 °C and the Effect of Morpholine and Dimethylamine on the Surface Charge of Magnetite at 150-250 °C, EPRI Report 1003179, EPRI, Palo Alto, CA, 2002.
[10] J.M. Riddle, G.D. Burns, L.J. Cain, Chemistry Control with Morpholine at Beaver Valley Power Station, Report NP-4623, Electric Power Research Institute, Palo Alto, CA, 1986.
[11] D.M. Shenberger, J.D. Zupanovich, J.L. Walker, N.W. Nolan, Loop testing of alternative amines for all-volatile treatment control in PWRs, Report TR-100756, EPRI, Palo Alto, CA, 1992.
[12] J.W. Cobble, P.J. Turner, PWR advanced all-volatile treatment additives, by-products, and boric acid, Report TR-100755, Electric Power Research Institute, Palo Alto, CA, 1992.
[13] P. Wang, A. Anderko, R.D. Young, A Speciation-Based Model for Mixed-Solvent Electrolyte Systems, Fluid Phase Equilibria, 203 (2002) 141-176.
[14] P. Wang, R.D. Springer, A. Anderko, R.D. Young, Modeling Phase Equilibria and Speciation in Mixed-Solvent Electrolyte Systems, Fluid Phase Equilibria, 222 (2004) 11-17.
[15] P. Wang, A. Anderko, R.D. Springer, R.D. Young, Modeling Phase Equilibria and Speciation in Mixed-Solvent Electrolyte Systems: II. Liquid-Liquid Equilibria and Properties of Associating Electrolyte Solutions, Journal of Molecular Liquids, 125 (2006) 37-44.