SOFTWARE | OLI STUDIO | CORROSION ANALYZER
Corrosion prediction software
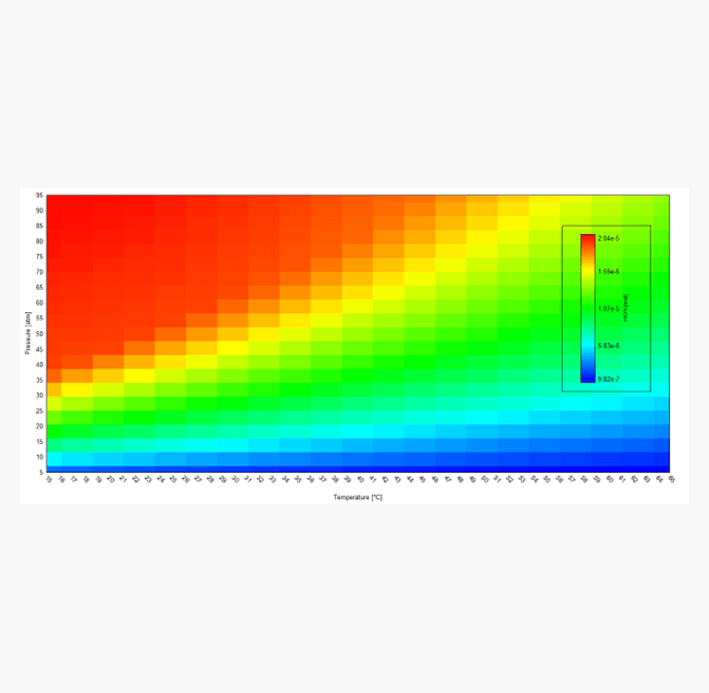
Advanced corrosion simulation software to determine the causes of corrosion, empowering operators to take decisive action. Evaluate and implement preventive measures, from optimizing operating conditions to material selection. With OLI precision insights, ensure long-term asset integrity and operational success by mitigating risks.

First-principles corrosion simulation software