In recent years, there has been a significant surge in the utilization of lithium-ion batteries (LIBs) owing to their remarkable energy density, minimal memory effect, and low self-discharge. Consequently, the widespread adoption of LIBs has sparked interest in the recycling of used batteries, driven by both economic incentives and environmental conservation requirements. The primary objective of recycling is to reclaim the constituent elements of depleted batteries to facilitate their reuse in battery production. Notably, cobalt, nickel, and lithium, which are primarily found in the cathode, are the main focus for recovery due to their substantial economic value. However, the challenge with LIB production and recycling lies in striking a balance between effectiveness and environmental sustainability.
Gaining profitability and a competitive advantage in lithium production can be achieved through enhancing speed, reducing costs, and finding methods to increase yield, purity, and production efficiency. Similar to other process industries, a strategic approach to gain a competitive edge is to develop engineering design schemes using mathematical modeling and principles of chemistry. This enables the exploration of innovative techniques and solutions to optimize the production process.
Process simulation difficulties
Different chemical processes are utilized for extracting lithium from various sources, including salars, lithium-bearing minerals, lithium-bearing clays, recycled batteries, oilfield produced water, hydrothermal deposits, and seawater. To achieve efficient and sustainable production, it is essential to comprehend the chemical mechanisms involved in each process. This understanding helps optimize yields, reduce operational costs, and ensure compliance with environmental regulations. Until recently, process simulation tools only partially addressed the optimization of lithium production schemes. They lacked rigorous chemistry calculations necessary to optimize yield and purity in the complex and highly reactive nature of lithium chemistry. Accurate solubility calculations at high salinity and simulation of the complex chemistry of double salts were missing features in major flowsheet simulator systems. Consequently, the industry relied on empirical approaches, known as “bucket chemistry,” which provided rough and imprecise characterizations of lithium process behavior.
OLI Systems electrolyte thermodynamics
Thermodynamic simulations play a critical role in optimizing conditions, maximizing yields, and reducing costs in lithium process techniques. OLI Systems, Inc., a global leader in electrolyte chemical technology, offers a distinctive advantage by overcoming the limitations of simplistic “bucket chemistry” approaches. It provides rigorous chemistry simulations that are essential for lithium processes. Through OLI’s MSE framework [1] integrated into the OLI Software Platform V11.5, OLI Systems incorporates the necessary chemical species and conducts comprehensive thermodynamic property calculations. This empowers researchers and engineers to engage in scientifically grounded, data-driven simulations that are reliable and accurate. OLI Systems’ framework exhibits robustness and adaptability, enabling precise and rigorous chemistry simulations for complex and interactive lithium chemistries.
The OLI Systems lithium chemistry update
In 2012, OLI Systems initiated the analysis of the chemistry involving lithium and potash. After 10 years, as release of OLI Software Platform V11.5, a comprehensive model encompassing – Li-Mg-Na-K-Ca-SO4-CO3-Cl-OH-NO3-H2O was released. OLI’s simulation capabilities include:
- Sulfate – chloride systems (Li-Na-K-Mg-Ca-Cl-H2O, Li-Na-K-Mg-Ca-SO4-H2O, and mixed Li-Mg-Cl-SO4-H2O, Li-Ca-Cl-SO4-H2O).
- Hydroxide and carbonate systems (Li-Na-CO2-OH-CO3-Cl-H2O)
- Nitrate systems for energy storage (Li-Na-Mg-Ca-NO3-H2O)
- Li hydrometallurgical processing, purification, and recycling (LiF, Li-Ni-Co-Mn-NH4-Cl-SO4)
- Others such as lithium borate systems, acid leaching systems (Li-H-Cl-SO4-H2O), as well as transport-properties in a battery.
The following sections show the model simulation of the complex systems related to lithium chemistry.
Strong inorganic acids such as HCl, H2SO4, and HNO3 can be used as leaching for LIBs [2]. Among these acids, H2SO4 has the lowest price and high availability, and it has shown good results for industrial applications [3]. But even the ternary system, Li2SO4-H2SO4-H2O, is roughly in agreement among experimental data up to 80 wt.% H2SO4 and there is a disagreement regarding the solid phase around 55 wt.% H2SO4.
Figure 1 shows that the OLI’s MSE model reliably predicts the ternary system Li2SO4-H2SO4-H2O. At 30 °C, there is no change of solid phase around 55wt.% H2SO4 although the water activity of H2SO4 at high concentration could be low enough to expect anhydrous lithium sulfate. As H2SO4 concentration increases more, the solid phase changes into Li2SO4∙H2SO4. On the other side, at a certain range concentration of H2SO4 (<40 wt.%), the solubility of Li2SO4 increases as the concentration of H2SO4 decreases. The results from the MSE model provide insights and a theoretical foundation for the separation of lithium and for studying its processing and recycling process.
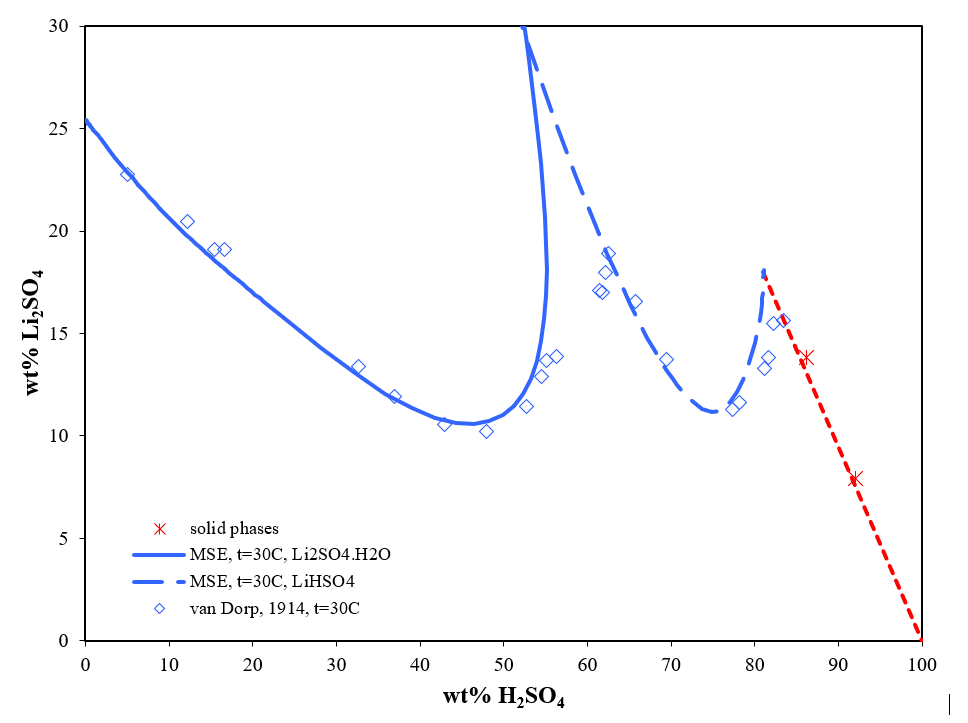
Figure 1. Ternary system Li2SO4-H2SO4-H2O at 30 °C
Metastable equilibrium is a ubiquitous phenomenon in nature, encompassing a wide range of natural systems. It is well established that metastable equilibrium bears a closer resemblance to the natural conditions of evaporation, thereby enabling an objective representation of the evaporation and crystallization behaviors of brine in the natural environment. However, despite the abundance and value of oilfield brine resources, their potential utilization has been constrained by the absence of comprehensive metastable phase diagrams and physiochemical property data. Therefore, the utilization of metastable phase equilibrium provides a means to comprehensively demonstrate the interaction between brine and minerals, as well as to unveil the intricate processes of salt crystallization.
The ternary system (Li2SO4-K2SO4-H2O) is such a system at 0 °C although the stable phase equilibrium at some other temperatures as reported. Figure 2 shows that the OLI’s MSE model reliably predicts the ternary system Li2SO4-K2SO4-H2O. From 0 °C to 100 °C, the model accurately predicts the metastable phase diagrams with two invariant points, three univariant solubility curves, and three metastable crystallization regions, corresponding to lithium sulfate monohydrate, double salt (Li2SO4∙K2SO4) and K2SO4. A comparison of the metastable phase diagrams at all temperatures shows that the area of K2SO4 is decreased obviously whereas the areas of double salt and Li2SO4∙H2O increase with increasing temperature, and no solid solutions were found.
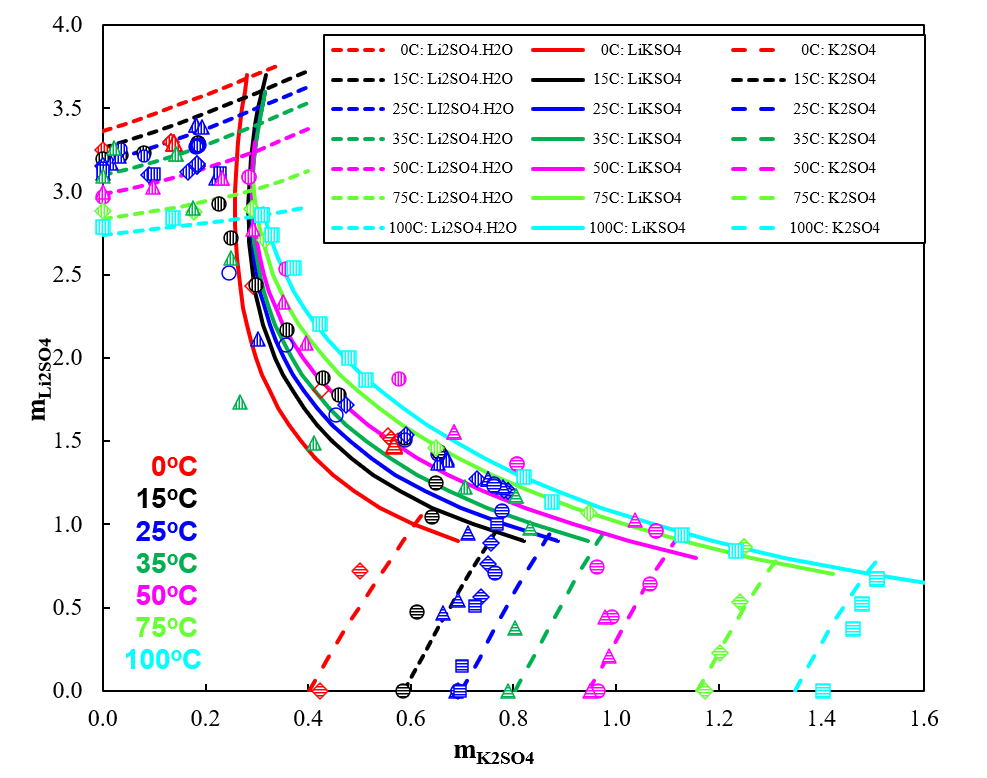
Figure 2. Ternary system Li2SO4-K2SO4-H2O
Salt-lake brine, which has high concentration of lithium, potassium, and boron, is a very important liquid mineral resource. It is important and practically significant to study the phase equilibrium and phase diagram in order to exploit the salt-lake resources. Furthermore, the water salt phase equilibrium provides the basic information about the evaporation, concentration, and crystallization of the salts. Understanding the salts solubilities can help design the metastable equilibrium and utilization of the salt-lake brine.
The salt-water quaternary system Li2SO4 – Na2SO4 – K2SO4 – H2O is one of the most important subsystems of the sulfate-type salt-lake brine. Figures 3 and 4 show the solubility diagram of this quaternary system Li+, Na+, K+ // SO42- – H2O at 15 °C. Both plots clearly show there are 6 invariant points, 12 univariant curves and 7 areas of crystallization field. In addition to simple salts Li2SO4∙H2O, K2SO4, Na2SO4∙10H2O, there are four double salts Li2SO4∙ K2SO4, 3K2SO4∙ Na2SO4, 2Li2SO4∙ K2SO4∙Na2SO4, and Li2SO4∙3Na2SO4∙12H2O.
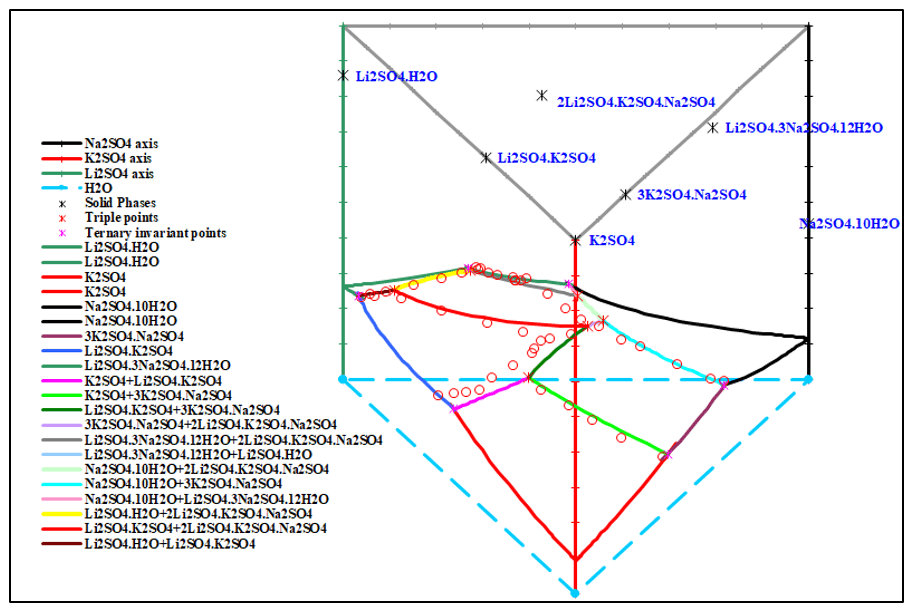
Figure 3. 3D diagram of the quaternary system Li+, Na+, K+ // SO42- – H2O at 15 °C
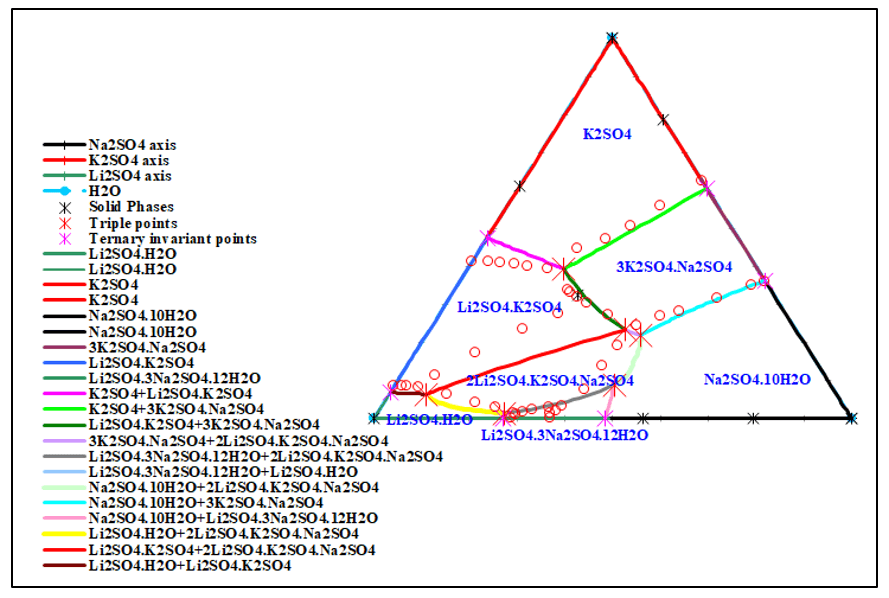
Figure 4. Jänecke diagram of the quaternary system Li+, Na+, K+ // SO42- – H2O at 15 °C
It also could be found that in figure 3 and 4, the crystallization field of salt Na2SO4∙10H2O is the largest whereas that of double salt Li2SO4∙3Na2SO4∙12H2O is the smallest field, which shows that sodium sulfate has smaller solubility in water than lithium sulfate under the coexisting ions and cand be easier to sperate through evaporation.
Another system for the extraction of valuable resources from salt lakes is Li2SO4 – K2SO4 – MgSO4 – H2O. It is essential for extracting salts from natural brines and clarifying formation and evolution of salt lakes containing salts.
Figure 5, 6, 7 show the phase diagrams of quaternary system Li+, K+, Mg2+ // SO42- – H2O at 25 °C. It contains six solubility branches and five crystallization fields corresponding to K2SO4, Li2SO4∙H2O, MgSO4∙7H2O, and double salts, Li2SO4∙K2SO4 and K2SO4∙MgSO4∙6H2O. The crystallization field of the double salt Li2SO4∙K2SO4 is the largest, whereas that of Li2SO4∙H2O is the smallest.
The prediction matches the experimental data very well.
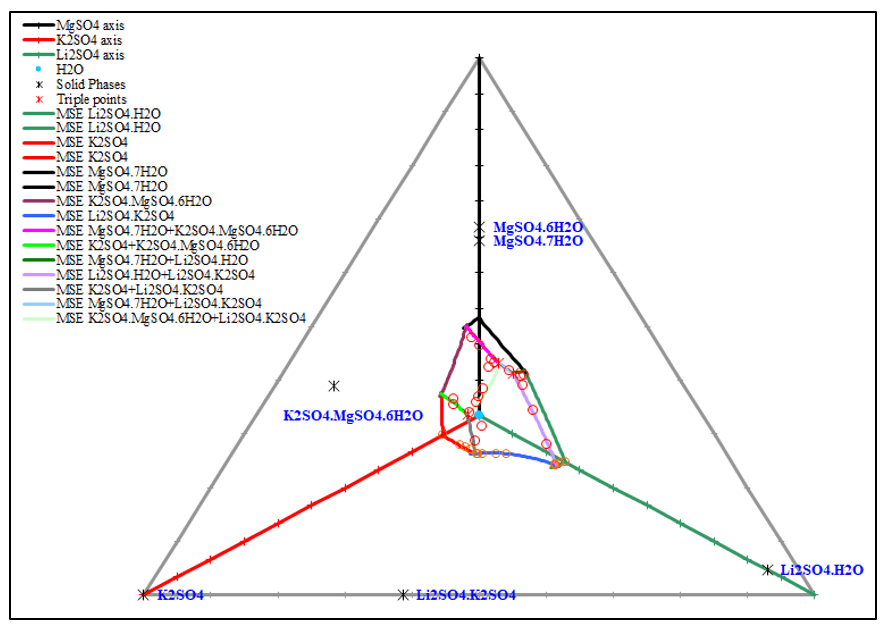
Figure 5. 3D diagram of the quaternary system Li+, K+, Mg2+ // SO42- – H2O at 25 °C
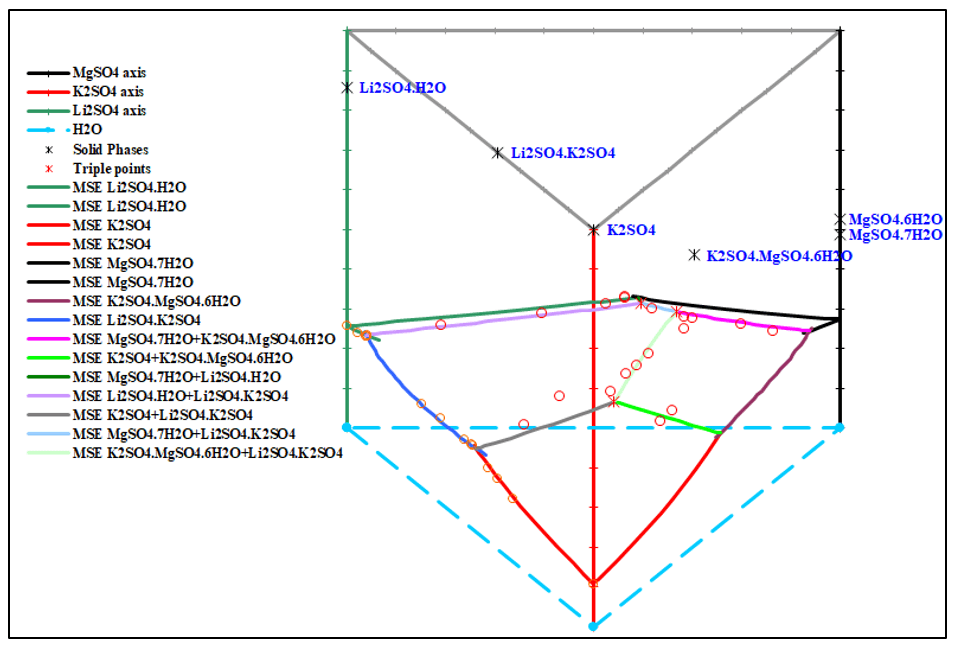
Figure 6. 3D phase diagram of the quaternary system Li+, K+, Mg2+ // SO42- – H2O at 25 °C
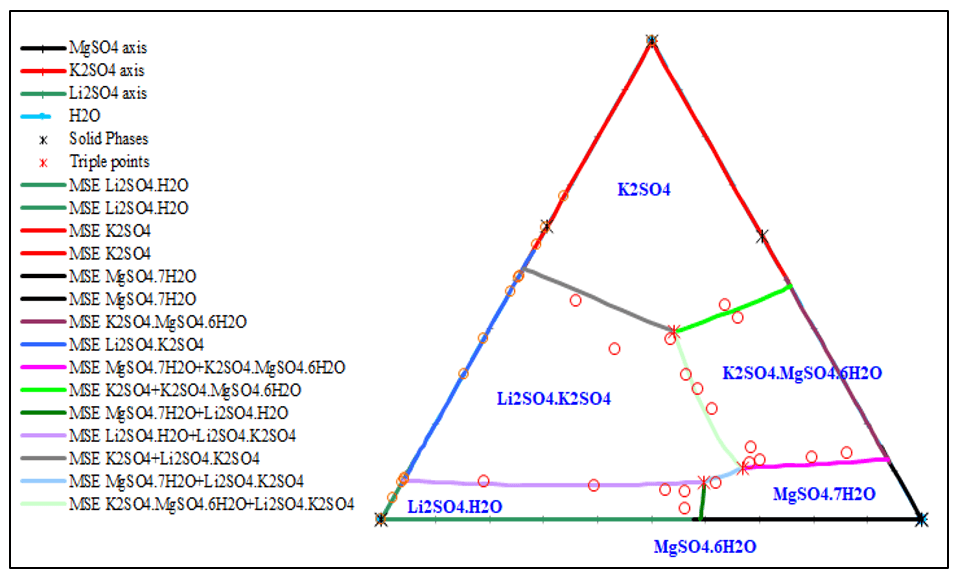
Figure 7. Jänecke diagram of the quaternary system Li+, K+, Mg2+ // SO42- – H2O at 25 °C
What tools are available for predicting lithium chemistry?
After more than 10 years of focus on lithium chemistry, OLI now can provide engineers with the ability to design more efficient processes, and to predict more closely than ever before the yield and purity of their products.
OLI Systems’ simulation packages, which are based on the MSE model, are available in OLI Studio V11.5 and OLI Flowsheet: ESP V11.5. A special databank is available to maximize the accuracy of modeling the lithium-bearing systems discussed above. This databank will be incorporated in version 12 of the software and, in the meantime, is available from OLI upon request.
Contact OLI at www.olisystems.com/contact-us for more information or to schedule a meeting with an OLI expert.
References
- [1] Wang P., Anderko A., Young R. D., “A Speciation – Based Model for Mixed – Solvent Electrolyte Systems”, Fluid Phase Equilibria, 203, (1-2), 141-176, 2002.
- [2] Mossali, N. Picone, L. Gentilini, O. Rodriguez, J.M. Perez, M. Colledani, J. Environ. Manage. 264,2020.
- [3] Song, Y.; Xie, B.; Song, S.; Lei, S.; Sun,W.; Xu, R.; Yang, Y. Regeneration of LiFePO4 from spent lithium-ion batteries via a facile process featuring acid leaching and hydrothermal synthesis. Green Chem. 2021, 23, 3963.

