It’s fun watching a new market find its commercial footing. In the early 1900’s there were hundreds of car manufacturers seeking their fortune. Fast forward 120 years, history is repeating itself. There are hundreds of innovative startups developing new technologies for EV cars. These companies tinker in lithium production, battery materials (anodes, cathodes, electrolytes), and the individual technologies that make the engineering possible.
A key growth area is technology to extract lithium from geologic fluids, salars, geothermal brines, and oilfield produced brines. Clever startups, universities, and university-to-startup groups have dusted off fifty-year-old chemistry to capture a piece of this market. The chemistry is lithium adsorption on using ion sieves (LIS) and LiCl adsorption using layered double hydroxide (LDH) intercalation. These groups are synthesizing, doping, functionalizing, applying charge, varying particle size and scaffolding material, all in efforts to optimize capacity, selectivity, reactivity, and durability. Keeping up with the journal articles is in itself a part-time job.
Collaborative innovations, advancing simulation tools for next-gen lithium extraction.
We at OLI have the good fortune of chatting and working with many of these entrepreneurs and the energy they bring is infectious. That energy in-turn, motivates and challenges us to produce better tools for their work. In our case, it’s about providing software tools that simulate the behavior of their LIS and LDH materials in a process environment. The challenge for us is that the material produced by the different groups vary in process characteristics. As such, we cannot develop a single material in our database to cover all products. So, we need to make available a dynamic database, one that allows for different selectivity’s, adsorption capacity, material solubility (when applicable), and adsorption/desorption reaction rates. The net effect is our own evolution in providing Li-extraction simulation.
Five years ago, we offered an empirical approach where the user entered the adsorption/desorption efficiency, and the software calculated a basic material balance. Soon after, we added a little more rigor by creating LIS materials with a defined adsorption capacities and selectivity coefficient for each cation. This didn’t work entirely, because a material with a single adsorption site would not show exclusivity to lithium and hydrogen. So, the next LIS material was made up of two exchange sites, one specific to Li/H and one that allowed all cations to adsorb. While this satisfied the exclusivity criterion, it still lacked the realistic effects of adsorption and desorption rates. The latest version now includes “fitted” reaction rates using lab or plant data. This combination we think now give users the ability to insert any LIS material in their process design to test plant performance as process conditions change.
The material getting heightened interest now are LDH. We first created a database about three years ago, using scant information available from literature. The material was a pseudomolecule (essentially unreactive to anything but LiCl) that calculated using molecular adsorption, the uptake. However, it really couldn’t mimic desorption into the low saline wash waters. More recently, we moved to a reactive LDH material based on Al(OH)3 properties and instead of relying on adsorption. We created the base LDH and separate LiCl-LDH phases.
“From early LDH databases to reactive materials, our journey mirrors the industry’s quest for efficient, cost-effective lithium extraction”.
We then used concentration-based forward rate expressions to predict uptake rates / capacity and then a salinity-dependent reverse rate expression to predict the desorption rate and extent. This represents our current capabilities.
While the materials these companies create, and our efforts to keep up with their advances will continue for some time, the ultimate goal is still the same. How can our industry extract more lithium with fewer impurities, using less chemical, and at a lower cost. That is where OLI will step up to the plate; to answer questions like what pH, contact time, brine loading, temperature, etc., will give the best results. We’re learning every day from these very users we help, and are bringing the technology to them as quickly as we can.
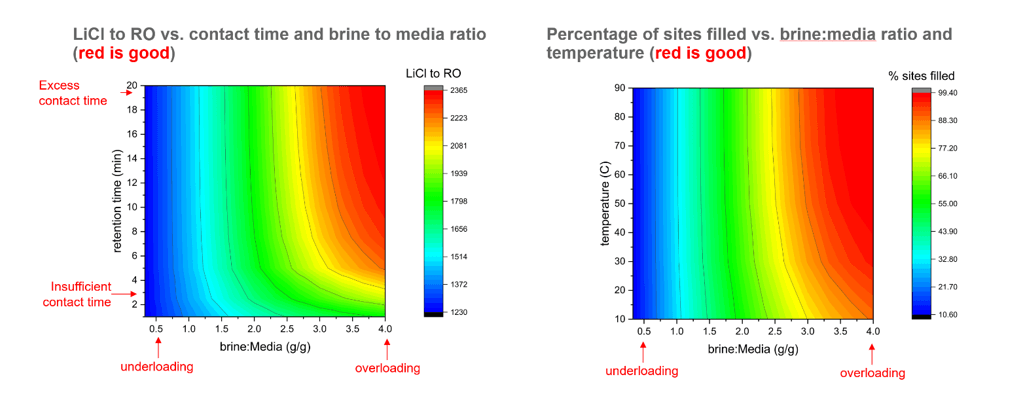
Figure: Optimization study on a published LDH material. We tested the effects of retention time, temperature and brine to media loading (g/g) on the LiCl product sent to the RO unit (left) and the media utilization for the adsorption cycle (right). Areas in red are the optimal conditions.

