Lithium-ion batteries (LIBs) are widely used in the electronic industry and energy storage due to the favorable characteristics of high energy density, long life cycles, high working voltage, no memory effect, low self-discharge and safe handling [1]. Recycling of spent LIBs is critical from an environmental and economic standpoint. One of the most widely used LIB cathodes including lithium cobalt oxide (LCO) contains high concentrations of heavy metals that if not properly treated could be harmful to the environment. Additionally, recycling can contribute to sustainable supplies for critical metals such as lithium and cobalt for which demand is expected to outstrip supply [2].
The recovery methods of spend LIBS can be divided into hydrometallurgy, pyrometallurgy and combination methods. During pyrometallurgical recycling, lithium is usually lost in the slag, and the elevated temperatures (900 – 1500˚C) required are associated with high energy costs and carbon dioxide emissions [3]. Hydrometallurgy is the most widely utilized method in industrial application. Generally, the cathode materials are separated and enriched by a series of pretreatment processes. Then, valuable metals are leached using strong acid solutions as leaching agents such as HCl, HNO3, H2O2 and H2SO4. Lastly, the salt solutions of Co, Ni and Mn are obtained by purification and solvent extraction. However, due to the penetration of leaching residue into the soil and ground water and emission of toxic gases such as Cl2, SOx and NOx, they are harmful to environment and human health [4].
In order to overcome the abovementioned drawbacks, effect of organic acids on the recovery of lithium and cobalt from spent Lithium-ion batteries have been widely investigated. Biohydrometallurgy is a subfield of hydrometallurgy that has the potential for reduction in chemical and energy consumption. Gluconic acid is one mild organic that has favorable characteristics such as being noncorrosive, nonvolatile and nontoxic [5]. Its production process is green, and it is less harmful to the environment. This acid is not only miscible with water but is also biodegradable. The existence of a carboxyl group adjacent to a hydroxyl group increases the acidity of the acid which provides great reaction versatility.
Process simulation deficiencies
As LiCoO2 is used as cathode, the dissolution of Li+ is accompanied by the formation of a sparingly soluble product, Co(OH)3. In the presence of ferrous ions, redox transformations may lead to the formation of hydroxides depending on pH. It is important to understand the solubility of the hydroxides due to incongruent dissolution.
On the other hand, part of the complexity of understanding the leaching reactions occurring in the recycling of LIB waste is the complex set of reactions involving Co2+, Mn2+, Ca2+, Mg2+, Zn2+, Fe2+, Fe3+ with gluconic acid. The complexation behavior has to be incorporated in the model because it is an important factor in determining the solubility in addition to pH effects. Typically, complex formation increases the solubility of species such as hydroxides. However, gluconic acid can also form solid metal gluconate species, which themselves provide a limit of solubility. Additional factors, such as the leaching acid concentration, reaction temperature, and liquid-solid mass ratio, will also affect the bioleaching process.
Understanding the chemical mechanisms of each reaction enables efficient and sustainable production, helping to recover high value-added metals while lowering operation costs, maintaining environmental compliance, optimizing pulp density and leaching duration, and improving industrial adoption of this new biotechnology.
OLI Systems sustainable bioleaching of LIB recycling process
Thermodynamic simulations are essential for achieving optimum conditions, maximizing yields, and lowering costs in bioleaching of LIBs recycling process techniques. OLI Systems is uniquely positioned for LIB recycling process and supply the missing mission-critical rigorous chemistry simulation for bioleaching of LIB recycling. OLI’s MSE framework [6], which is incorporated in OLI Software Platform V11.5, contains the requisite chemical species and calculates the thermodynamics properties that are necessary to enable science-based, data-driven, and reliable simulations. OLI Systems’ framework is sufficiently robust and extendible to deliver rigorous and accurate chemistry simulation for any complex and interactive chemistry of interest.
Can we reliably predict the bioleaching of LIB using OLI Software Platform?
A major subset of OLI Systems’ bioleaching LIB recycling chemistry initiative is complete with the release of OLI Software Platform V11.5. OLI’s simulation capabilities include:
Hydroxides of cobalt and iron in both II and III oxidation states
The solubility of the hydroxides is important because incongruent dissolution may lead to the formation of hydroxides and, also, redox transformations may lead to the formation of hydroxides depending on pH. The pH ranges in which the hydroxides precipitate are particularly important. Different cations have multiple aqueous species in the solution under different pH range.
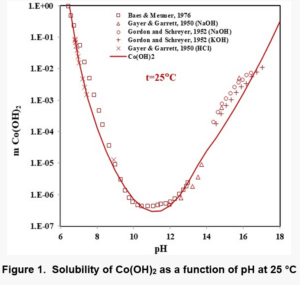
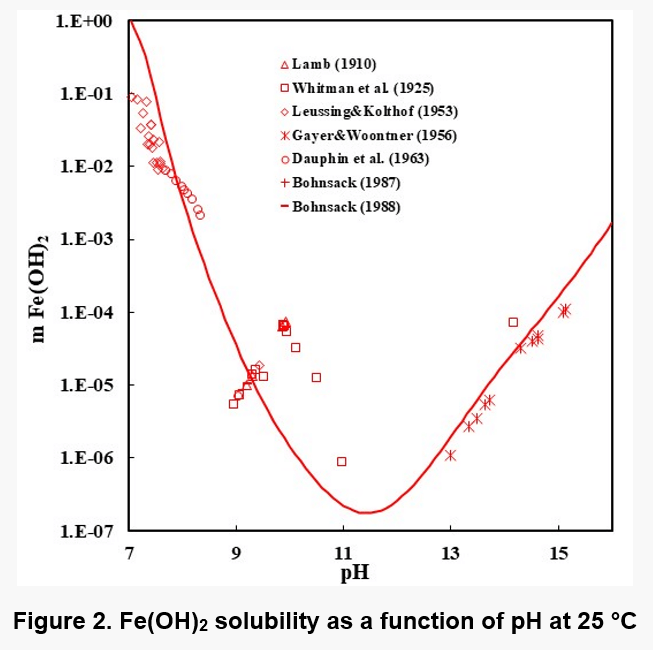
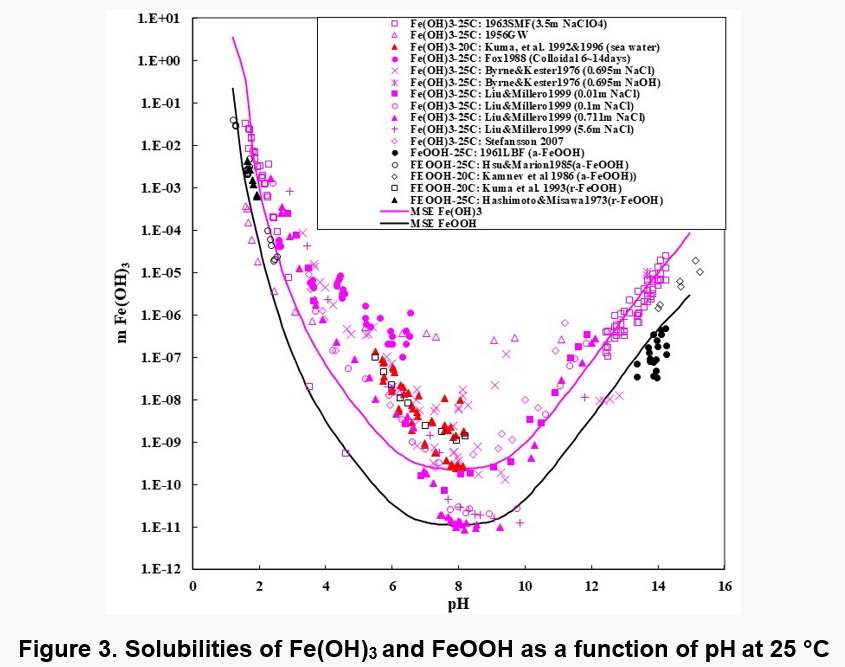
Figures 1, 2, and 3 show the solubility of Co(OH)2, Fe(OH)2, and Fe(OH)3. There are no experimental solubility data for Co(OH)3, but the solubility can be predicted based on thermochemical data.
In the case of Fe(OH)2, the model takes into account the Fe+2, Fe(OH)+1, HFeO2-1 and FeO0 species in the solution when ferrous hydroxide dissolves in water. Analogous species exist in the case of Co(OH)2. In the case of Fe(OH)3 solutions, the aqueous species are HFeO20, Fe+3, FeO2-1, FeOH+2, FeO+1 and Fe2(OH)2+4. The Fe(III) shows more complexation.
It is noteworthy that the solubility patterns of Fe(OH)2 and Co(OH)2 are quite similar. On the other hand, the solubility of Fe(OH)3 is much lower. Therefore, unlike Fe(OH)2 or Co(OH)2, Fe(OH)3 can precipitate in fairly acidic solutions.
Completed complexation equilibrium of relevant cations with gluconic acid
Table 1 lists the complexes that have been identified for all relevant cations (i.e., Co(II), Fe(II), Fe(III), Ca(II), Mg(II), and Mn(II)) with gluconic acid. The complexation behavior is clearly explained in the model.
Table 1. Complex species formed by the relevant cations with gluconic acid.
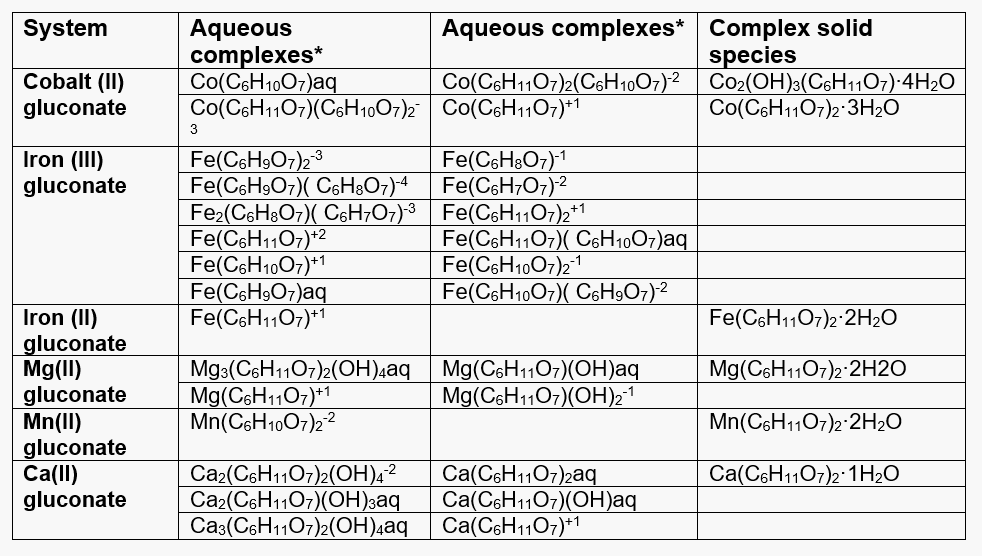
*The ligands are defined as:
C6H12O7 – undissociated gluconic acid
C6H11O7– – ion after the dissociation of the carboxylic group proton
C6H10O72- – ion after the dissociation of the carboxylic group proton and one hydroxyl group proton
C6H9O73- – ion after dissociation of two hydroxyl group protons
C6H8O74- – ion after three hydroxyl groups dissociate
C6H7O75- – ion after four hydroxyl groups dissociate
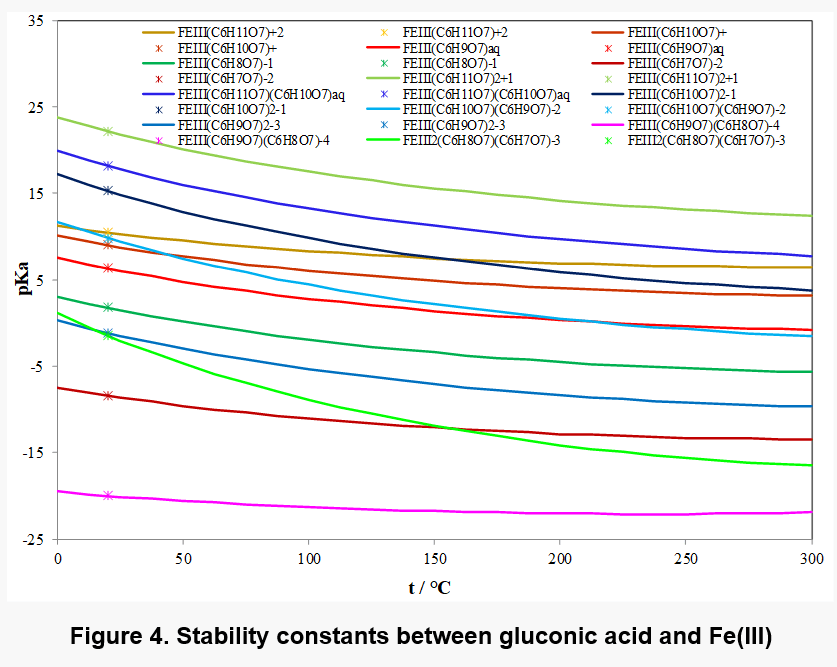
Fe(III) shows particularly strong complexation with gluconic acid and a variety of complex species has been identified. Depending on the concentration of ligand, complexes of Fe3+: D-gluconate stoichiometry 1: 1, 1:2 and 2:2 are formed. The deprotonation of the species is then dependent on the pH of the solution.
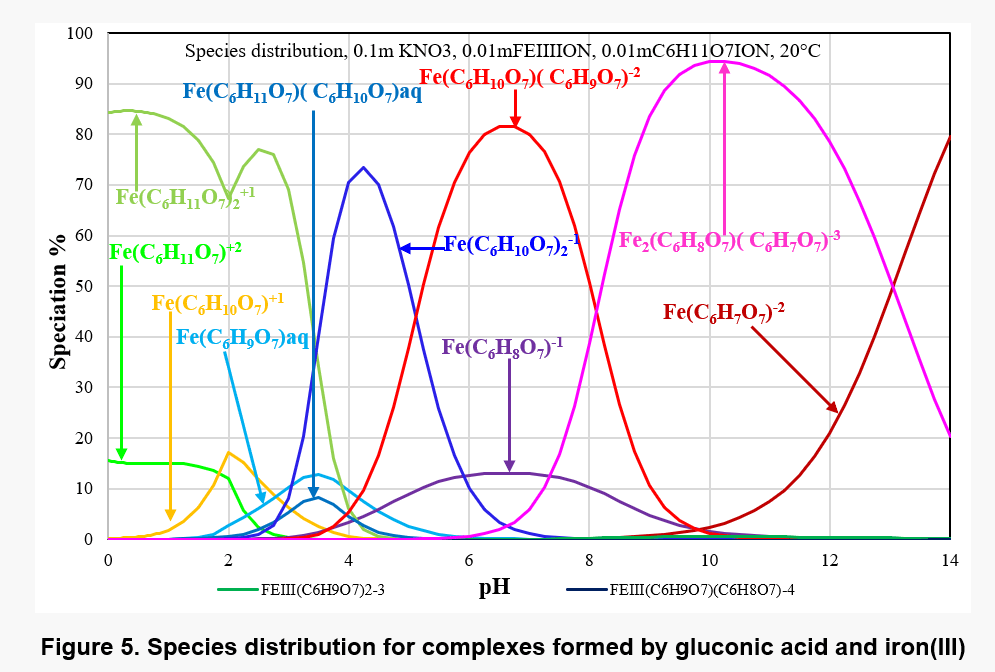
Figure 4 shows the equilibrium constants for the various complexes and Figure 5 shows the distribution of complex species as a function of pH in a solution containing 0.01 m Fe(III) and 0.01 m gluconate ions. Different main species appear in different pH range, for example, Fe2(C6H8O7)( C6H7O7)-3 is present in solution as the main complex species in pH range 8 to 13. In view of the limited solubility of Fe(OH)3, its solubility may be affected by the formation of these complex species.
Less is known about the complexation behavior of Fe(II). In this case, one solid complex species, Fe(C6H11O7)2·2H2O can precipitate in fairly concentrated solutions.
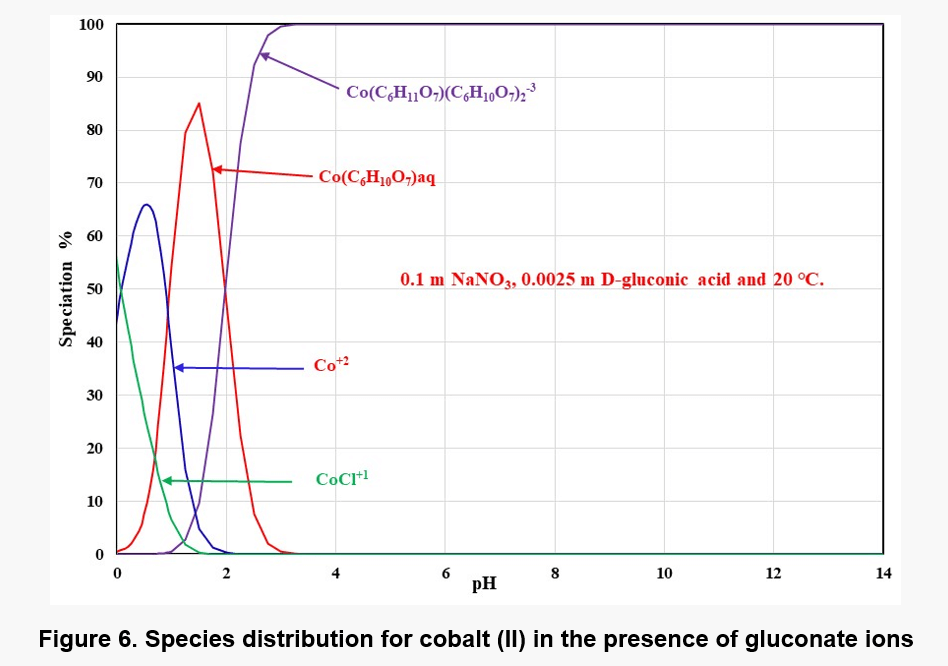
Except complexations, solid complex species also form with gluconic acid. Cobalt can form two solid complexes, i.e., Co(C6H11O7)2·3H2O and a basic complex species, Co2(OH)3(C6H11O7)·4H2O. The latter species is fairly sparingly soluble and, therefore, it can limit the solubility of Co(II) at some conditions. Figure 6 shows the distribution of Co – gluconate complexes as a function of pH at 0.1 m NaNO3, 0.0025 m D-gluconic acid and 20 ℃.
For completeness, the model also includes the complexation behavior of the ions that exist in the biological medium, i.e., Mg, Mn, and Ca. While the concentration of these cations is rather small, their presence may affect the overall material balances of complexation. All these cations can form solid species with gluconic acid. However, the solubility of these species is rather high (not listed here) and, therefore, they are not likely to form at any conditions related to the leaching process.
Solubility of the relevant phosphates of lithium, cobalt, and iron
The bioleaching medium contains a substantial amount of dissolved phosphate. The formation of REE phosphate was an expected phenomenon in biological media. Transition metals also form sparingly soluble phosphates although their solubility is not as low as that of REE phosphates. Therefore, we have performed an analysis of the solubility of cobalt and iron phosphates to be able to ascertain whether they can interfere with the bioleaching process. While lithium phosphate has a higher solubility, it has also been investigated to ensure that the interactions between lithium and phosphate ions in the solution are correctly accounted for.
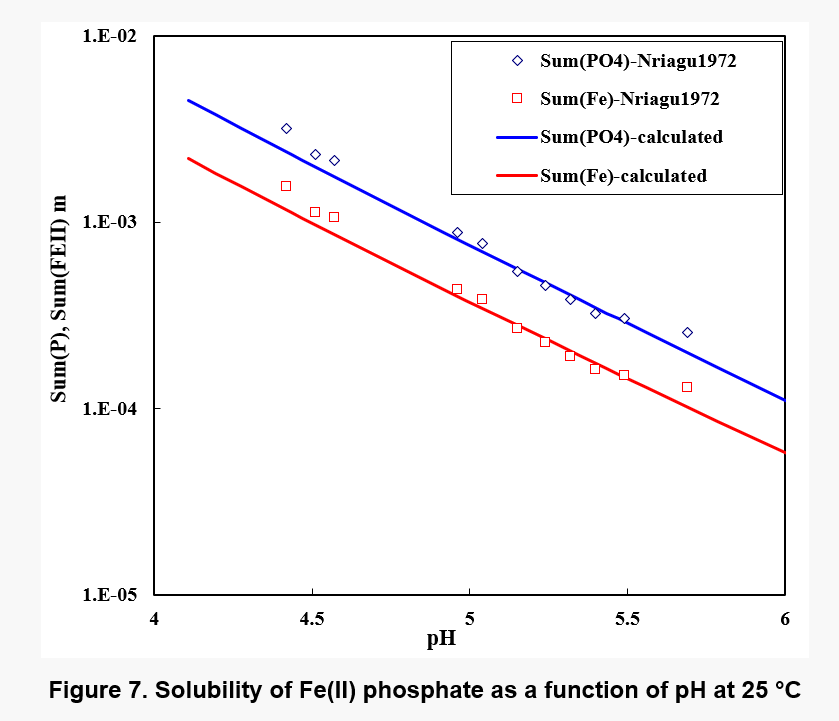
Figure 7 shows the solubility of Fe(II) phosphate. While the solubility is fairly low in the neutral range, it significantly increases in acid solutions. Nevertheless, precipitation of iron phosphate may be expected to bioleaching environments under some conditions.
Thermodynamic analysis of LiCoO2 bioleaching
The LCO was presumed to be contacted with the microbiological medium (Pkm which includes different concentrations of (NH4)2SO4, MgSO4, MnSO4, KCl, CaCl2 and KH2PO4) in the presence of gluconic acid, which exhibits chelation behavior in addition to impacting the pH; complexation of metals by gluconic acid was included in the modeling. FeSO4 was added in addition to LiCoO2 so that the molar ratio of Fe to Co is 1:1. Further, redox reactions was included in the model.
The results are shown in Figure 8. The redox reactions lead to a strong reduction of Co(III) to Co(II), accompanied by oxidation of Fe(II) to Fe(III). This leads to solubilization of Co. However, the solubilization of Co is strongly dependent on pH. At pH values up to ~3.5, most of Co is solubilized (cf. the pink line in Fig.8). This pH range can be obtained using 0.1 or 0.22 M gluconic acid as shown by the red arrows in Figure 8. At pH values above ~3.5, solid phases containing Co(II) begin to appear.
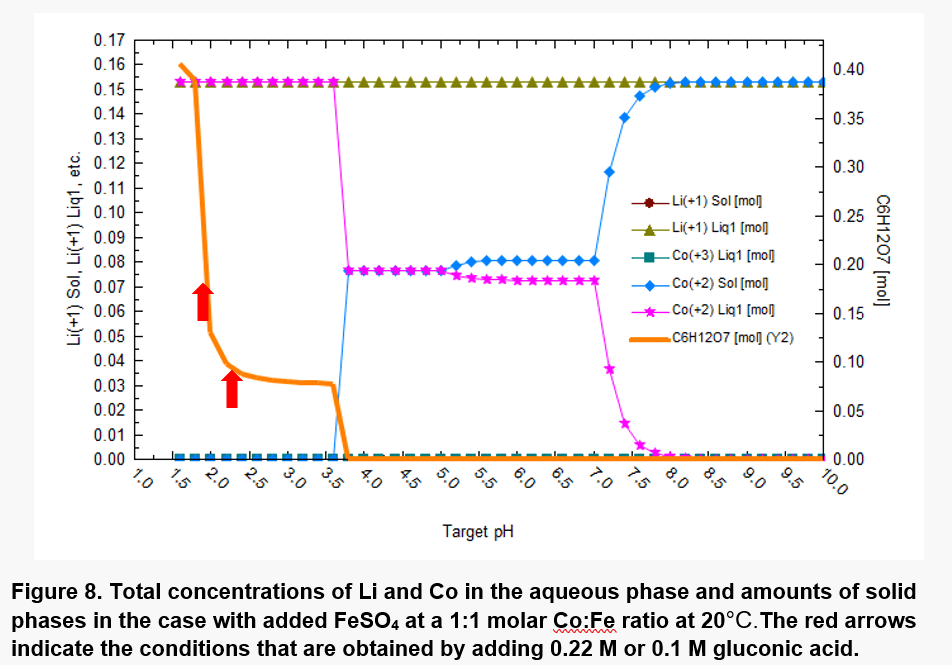
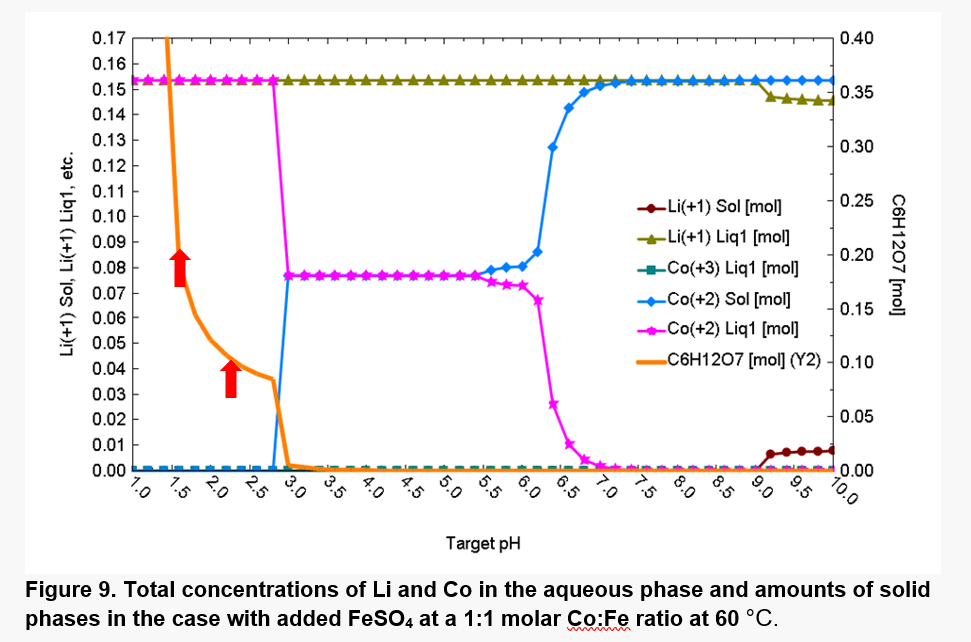
As shown in Fig. 9, at 60°C, most of Co is solubilized at pH values up to ~3.0 due to the shift in pH. The hydrolysis of the Fe(II), Fe(III), and Co(II) cations also strongly affect the acid-base equilibria.
Figures 8 and 9 have been generated on the assumption that the most thermodynamically stable phases will form, but some phases such as CoFe2O4 do not form for kinetic reasons. Figure 10 excludes some solid phases that are not likely to form at moderate conditions. Cobalt is then predicted to be mostly solubilized at pH values up to ~7, although a relatively small amount of precipitated Co(II) starts appearing at pH above ~5.
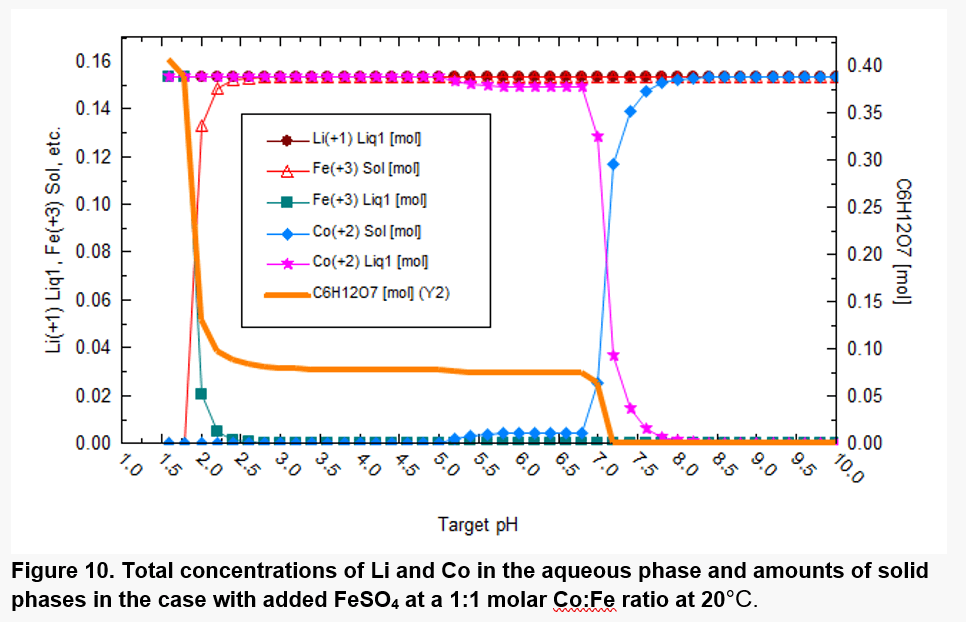
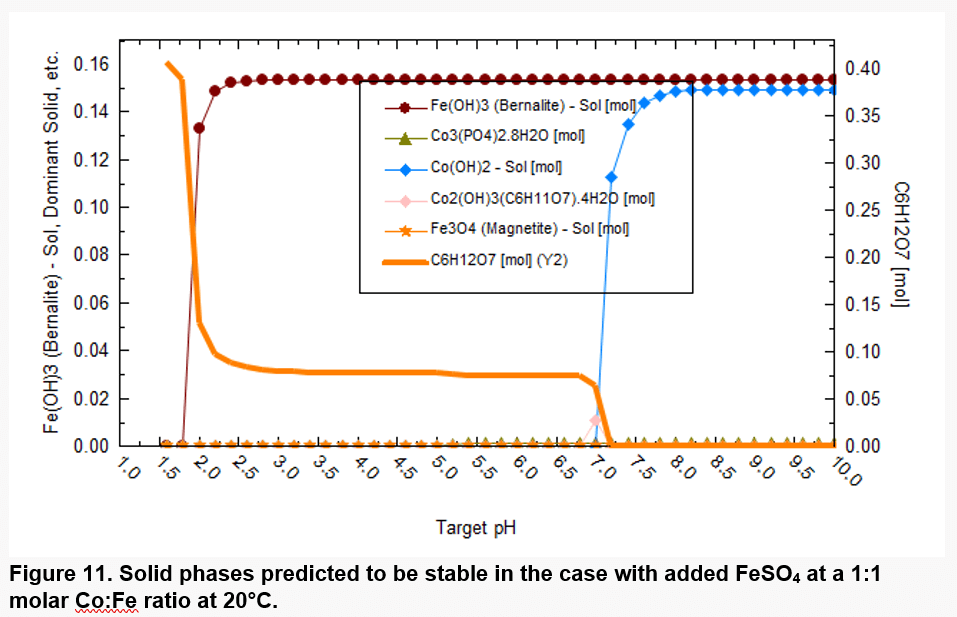
Figure 11 shows which solid phases are expected to be thermodynamically stable in a more realistic scenario. Co starts to precipitate at pH above ~7 in the form of Co(OH)2 and Fe precipitates in the form of only Fe(OH)3. Iron (III), which forms as a result of oxidation of Fe(II), has a strong tendency to precipitate. Fe(OH)3 is predicted to precipitate at pH above ~2 due to its low solubility. Thus, precipitation of Fe(III) is expected to accompany the dissolution of Co.
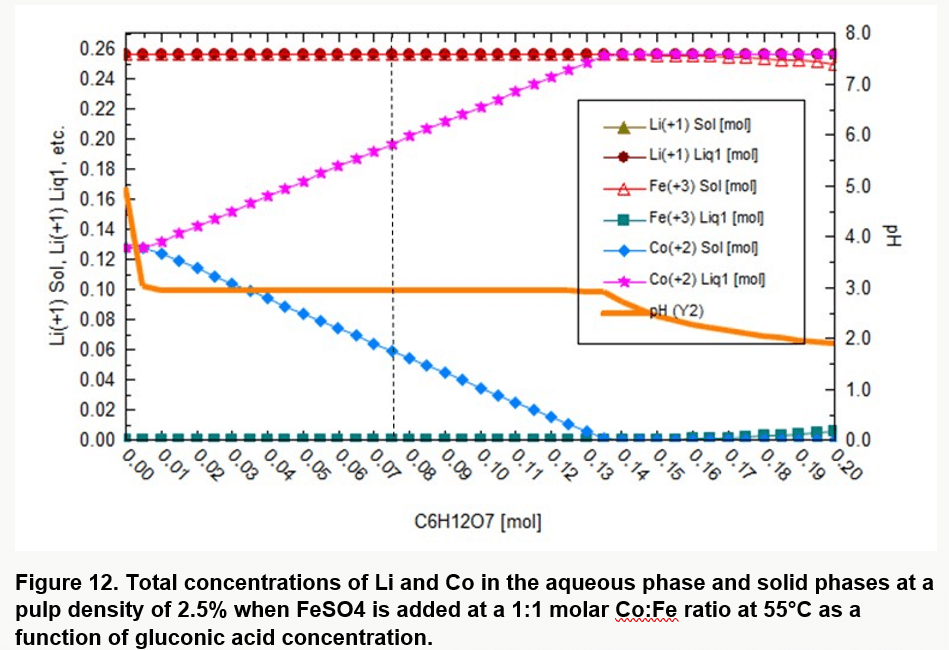
Pulp density is also an important factor in bioleaching LIBs. Low pulp densities mean larger volume of gluconic acid which increases cost. Thermodynamic simulations predicted further that for the selected pulp density and temperature, a gluconic acid concentration of 135 mM would be required to completely solubilize the Co (Figure 12).
What tools are available for bioleaching LIBs recycling process?
The OLI MSE framework is uniquely positioned to model this process, and MSE is a proven model that can accurately simulate complex, multi-component electrolyte systems. It provides engineers with the ability to design more efficient processes, and to predict more closely than ever before the yield and purity of their products.
OLI Systems’ simulation packages, which is based on the MSE model, is available in OLI Studio V11.5 and OLI Flowsheet ESP V11.5.
Contact OLI at https://www.olisystems.com/contact-us/ for more information or to schedule a meeting with an OLI expert.
References
- Arshad, F., Li, L., Amin, K., Fan, E.S., Manurkar, N., Ahmad, A., Yang, J.B., Wu, F., Chen, R.J., 2020. A comprehensive review of the advancement in recycling the anode and electrolyte from spent lithium-ion batteries. ACS Sustain. Chem. Eng. 8, 13527–13554.
- Xu, C.J.; Dai, Q.; Gaines, L.; Hu, M. M.; Tukker, A.; Steubing, B., 2020. Future material demand for automotive lithium-based batteries, Commun. Mater. 1.
- Kwon, O. S.; Sohn, I., 2020, Fundamental thermokinetic study of a sustainable lithium-ion battery pyrometallurgical recycling process, Resour. Conserv. Recycl. 158, 104809.
- Li, L.; Fan, E.; Guan Y.; zhang, X.; Xue, Q.; Wei L.; Wu, F.; Chen, R., 2017, Sustainable recovery of cathode materials from spent lithium-ion batteries using lactic acid leaching system, ACS sustain, Chem. Eng. 5224-5233.
- Golmohammadzadeh, R.; Faraji, F.; Rashchi, F., 2018, Recovery of lithium and cobalt from spent lithium-ion batteries (LIBs) using organic acids as leaching reagents: a review, Resour. Conserv. Recycl. 136, 418-435.
- Wang P., Anderko A., Young R. D., 2002, A Speciation – Based Model for Mixed – Solvent Electrolyte Systems, Fluid Phase Equilibria, 203, (1-2), 141-176.

