Importance of rare earths for the economy and strategic security
Rare-earth elements (REE), a family of 17 elements in the periodic table starting with lanthanum and ending with lutetium along with yttrium and scandium, are necessary components for more than 200 products across a wide range of applications. These applications include high-performance magnets used in wind turbines and generators, industrial automation, computers, audio systems, and automobiles, smartphones and devices, battery alloys, phosphors, high-performance metal alloys, automobile, and petroleum refining catalysts, polishing powders, glass additives, ceramics, defense-related and medical equipment. In 2019 alone, China manufactured $1 trillion worth of products from its rare earth material supplies. Due to increasing adoption of smart and environmentally sustainable technologies such as smartphones, watches, wind energy and electric vehicles, high growth rates are projected for the demand of some of the REEs (i.e., Nd, Eu, Dy, Tb, and Y). REEs are classified as critical materials in many countries, including the United States and the European Union. The future availability of REEs is likely to be constrained due to monopolistic practices of suppliers, availability of geological reserves, and environmentally unsustainable mining processes. For example, China dominates the world market of REEs minerals (over 90% of the supply, with about 30% of the reserves) and has previously increased export tariffs on REEs, drastically restricting their export quotas in 2009-2011 by about 40%. This has impacted entire industries and prevented the implementation of clean energy technologies. Recently, to curtail the supply risk, U.S. has started the “Round Top Project,” which holds the promise of making the United States largely self-sufficient in these critical minerals as the Round Top Mountain in Texas offers a 130-year supply. However, the development of sustainable environment friendly processes for producing high-purity rare earth oxides, their derivatives, and downstream materials require innovative solutions.
How OLI Systems is bringing novel environmentally friendly solutions to REEs processing industries?
Keeping the long term economic and strategic importance of the rare earth elements in mind, over the last eight years, OLI Systems Inc. has dedicated significant research effort to finding innovative solutions for REE separation and recycling processes based on rigorous scientific understanding. OLI Systems Inc. is also involved in the Department of Energy sponsored Critical Material Institute Hub, partnering with various universities and national labs including Rutgers, University of California, Davis, Idaho National Lab, and Lawrence Livermore National Laboratory, to outline next-generation processes such as bio-separation and adsorption of REEs, enhanced acid leaching of REEs from synthetic phosphogypsum and consumer electronic devices, biological wastewater treatment and recovery of REE. OLI Systems Inc. is committed to delivering the latest scientific innovations and solutions to the industry through its advanced process simulation software based on a state-of-the-art thermophysical properties databank. The thermophysical property databank exclusively offers chemistries of a wide variety of rare earth containing compounds needed for converting the ores, waste material streams, and electronic devices into industrially useful materials.
Importance of electrolyte thermodynamics for REEs
Although rare earth elements have become important, lack of knowledge about the fundamental properties of rare earth-containing compounds has the primary roadblock for designing a sustainable environment-friendly process with high reliability. In the very early stage, OLI had identified this shortcoming and established a thermodynamic modeling foundation for broad-based solution chemistry using its proprietary Mixed Solvent Electrolyte (MSE) model, which includes chlorides, hydroxides, phosphates, sulfates, and carbonates of REEs and various complexing agents. The thermodynamic model, available in OLI software V10, enables scientific, data-driven and reliable simulations that can improve process efficiency and sustainability.
Why are rare earth sulfates important?
The rare earth sulfates are key intermediary components and end products of various hydrometallurgical processes for rare earth production. Examples include recovery of rare earth elements from phosphate rock and clay minerals through sulfuric acid digestion of ores, recovery of rare earth elements from electronic waste using sulfuric acid leaching, and separation of rare earth elements through precipitation of sparingly soluble double sulfates of rare earths and sodium. Therefore, accurate estimations of the thermodynamic properties of rare earth sulfates and their double salts are essential for the optimal performance of various chemical processes involving those compounds.
Can we reliably predict the behavior of REEs in aqueous systems?
The MSE thermodynamic framework in the OLI software platform V10 can accurately estimate solubilities of REE sulfates over wide ranges of temperature in different solutions while identifying stable and meta-stable hydrated and anhydrous solid phases. For example, the following figure depicts the solubility of Ce2(SO4)3 in H2O. The MSE model accurately predicts the formation of three precipitating solids, i.e., ice, Ce2(SO4)3.12H2O, Ce2(SO4)3.9H2O, Ce2(SO4)3.8H2O and Ce2(SO4)3.4H2O, and identifies the transition temperatures between these solids. The calculated solubilities are in good agreement with the experimental data with the exception of the data points that are clear outliers.
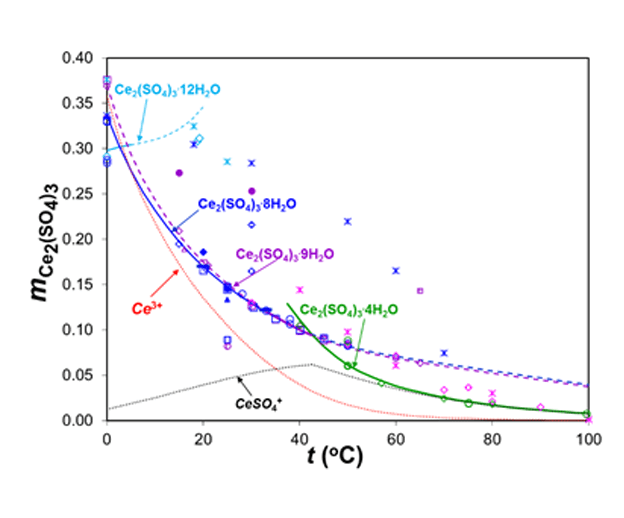
Solubilities in the Ce2(SO4)3-H2O system as a function of temperature
For separation processes, the relative solubility of the double salts is very important because it controls the selectivity of the separation. Although for some of the REE2(SO4)4-Na2(SO4)4-H2O systems no solubility data is available, the MSE model can reliably predict their solubilities, which agree with the overall trend. In the following figure, the solid circles represent the solubilities that were calculated for systems for which experimental data are available, whereas the open circles show the solubilities that were predicted using estimated parameters.
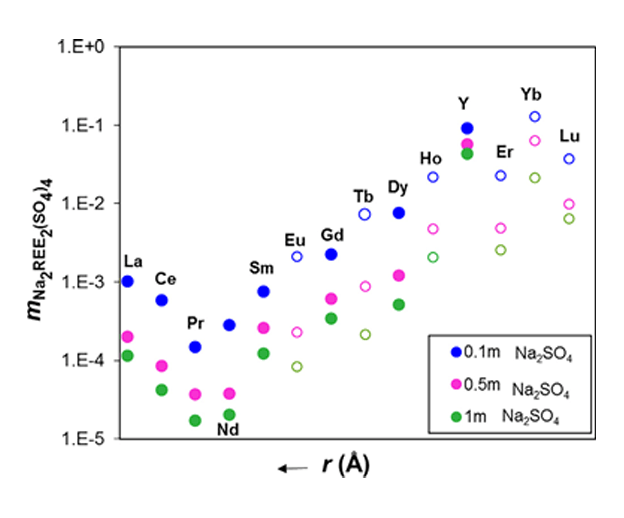
Solubilities of the Na2REE2(SO4)4.2H2O double salts as a function of crystal cationic radii in 0.1 m, 0.5 m and 1 m Na2SO4 solutions at 25 ºC
Apart from the solubilities, the MSE model is capable of calculating other thermodynamic properties of REE sulfates such as vapor-liquid equilibria and caloric properties (heat capacities, heat of dilution) which are used to analyze and optimize streams in a process. More details of our work on REE containing systems can be found in our published articles. [1,2]
What tools are available for predicting REE properties?
The OLI System’s thermodynamic property package which is based on the MSE model, is available in OLI Studio V10 and OLI Flowsheet ESP V10. For more updates on this project and the OLI System’s thermodynamic property package, its components and applicability, follow my researchgate page https://www.researchgate.net/project/A-broad-based-study-of-thermodynamic-properties-of-rare-earth-element-containing-compounds-for-industrial-separation-purposes.
Contact OLI at https:/www.olisystems.com/contact for more information or to schedule a meeting with an OLI expert.
References
- [1] Das, G., Lencka, M. M., Eslamimanesh, A., Wang, P., Anderko, A., Riman, R. E., & Navrotsky, A. Rare earth sulfates in aqueous systems: Thermodynamic modeling of binary and multicomponent systems over wide concentration and temperature ranges. The Journal of Chemical Thermodynamics, 2019, 131, 49-79
- [2] Das, G., Lencka, M. M., Eslamimanesh, A., Anderko, A., & Riman, R. E. Rare-earth elements in aqueous chloride systems: Thermodynamic modeling of binary and multicomponent systems in wide concentration ranges. Fluid Phase Equilibria, 2017, 452, 16-57

