Presently, lithium-ion batteries (LIBs) are widely used in the electronic industry and energy storage due to the favorable characteristics of high energy density, long life cycles, high working voltage, no memory effect, low self-discharge and safe handling [1]. Lithium cobalt oxide (LiCoO2) is the most common cathode material for commercial LIB due to its advantages of high charging cut-off voltage and high compaction density. Advances in the commercial development of lithium-ion batteries have spawned significant growth of demand for lithium (Li), cobalt (Co), manganese (Mn), and nickel (Ni). Due to the rapid increase in consumption of electronic products, the LIB consumption and demand sharply increase, thus increasing the production as well as end-of-life (EOL) waste. These spent LIBs contain a large quantity of heavy metals and organic chemicals which present a serious threat to ecological systems and human health. Conversely, they have a high economic value because they contain valuable metals such as lithium, cobalt, manganese, and nickel. Therefore, for waste management, resource conservation, to meet industrial needs and to reduce carbon footprint, the treatment and metal recovery from spent LIBs batteries has become imperative to support the circular economy and prevent environmental pollution. Hence, considerable attention has been paid to clean and efficient recycling of spent LIBs.
The recovery methods of spend LIBs can be divided into hydrometallurgy, pyrometallurgy and combination methods. Among them, hydrometallurgy is the most promising method in industrial application. The cathode materials are separated and enriched by a series of pretreatment processes. Then, valuable metals are leached using strong acid solutions as leaching agents such as HCl, HNO3, H2O2 and H2SO4. Lastly, the salt solutions of Co, Ni and Mn are obtained by purification and solvent extraction.
Process simulation deficiencies
Compared with other inorganic acids as acidic leaching agents, sulfuric acid has been the most widely used leaching agent with the help of hydrogen peroxide as a reducing agent for improving the solubility of cobalt, manganese, and nickel present in the cathode material. Also, sulfuric acid is currently the most promising acid for large-scale hydrometallurgical plants due to its low cost, effectiveness (low liquid/solid ratio required), and familiarity from ore refining processes. Part of the complexity of understanding the leaching reactions occurring in the recycling of LIB waste is the complex set of reactions involving Li+, Ni2+, Co2+, Mn2+, Ca2+, Mg2+, Zn2+, Na+, SO42-, as well as the interplay of leaching acid concentration, reaction temperature, and liquid-solid mass ratio. Understanding the chemical mechanisms of each reaction enables efficient and sustainable production, helping to recover high value-added metals while lowering operation costs and maintaining environmental compliance.
Until recently, process simulation has been able to address only some aspects of optimizing LIB recycling schemes. What was lacking was the rigorous chemistry calculations that are critical for yield and purity optimization for the highly reactive and complex LIB recycling chemistry. What is needed is accurate solubility calculations at high salinity, and also the simulation of the complex chemistry of double salts. These missing features made any chemistry predictions out of reach of all major flowsheet simulator systems
OLI Systems electrolyte thermodynamics in LIB recycling process
Thermodynamic simulations are essential for achieving optimum conditions, maximizing yields, and lowering costs in lithium recycling process techniques. OLI Systems, Inc., a global electrolyte chemical technology leader is uniquely positioned for LIB recycling process and supply the missing mission-critical rigorous chemistry simulation for LIB recycling. OLI’s MSE framework [2], which is incorporated in OLI Software Platform V11.5, contains the requisite chemical species and calculates the thermodynamics properties that are necessary to enable science-based, data-driven, and reliable simulations. OLI Systems’ framework is sufficiently robust and extendible to deliver rigorous and accurate chemistry simulation for any complex and interactive chemistry of interest.
Can we reliably predict the behavior of LIB recycling using OLI Software Platform?
A major subset of OLI Systems’ LIB recycling chemistry initiative is complete with the release of OLI Software Platform V11.5. OLI’s simulation capabilities include:
- Fundamental sulfate-chloride systems, fundamental hydroxide and carbonate systems.
- Lithium borate systems and the systems related to caliche sources and thermal energy storage.
- Systems related to Li hydrometallurgical processing, purification, and recycling; an example is shown in Figure 1.
- Miscellaneous systems containing lithium chloride with silica, methanol, ethanol, and formic acid
- Systems related to battery electrolytes, such as lithium hexafluorophosphate, lithium tetrafluoroborate, and lithium perchlorate in some pure and mixed carbonates.
Figure 1 shows the complex sulfuric acid leaching mixtures in LIB recycling process reaction system. OLI Software Platform V11.5 covers the key binary and ternary systems and shows the full extent of all necessary interaction parameters to bring the system to the highest degree of accuracy. Nine binary systems including sulfuric acid with water, and thirty-six ternary interactions are present for comprehensive simulation coverage.
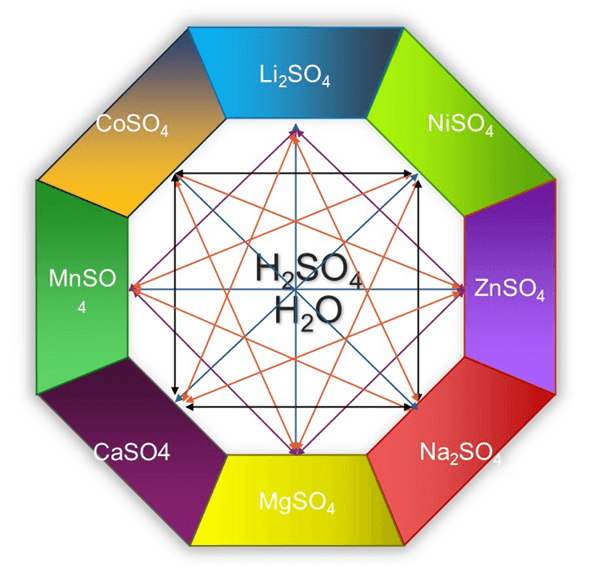
Figure 1. LIB leaching chemistry in sulfuric acid
Figure 2 shows the solubility behavior of CoSO4 in water as a function of temperature ranging from -5 to 220 °C and indicates a good agreement of the predictions with experimental data. Besides anhydrous CoSO4, three solid CoSO4 hydrates exist, with 1, 6, and 7 water molecules. Between 50 and 65 °C, the prediction shows three hydrate transitions. The solubility of CoSO4 in water increases until 65°C, then decreases at higher temperature. OLI Software Platform V11.5 not only predicts the solubility behavior of CoSO4, but also calculates other properties of CoSO4 solutions (activity of water and activity coefficient of aqueous CoSO4, vapor-liquid equilibria, enthalpies, heat capacities and densities of the solution)
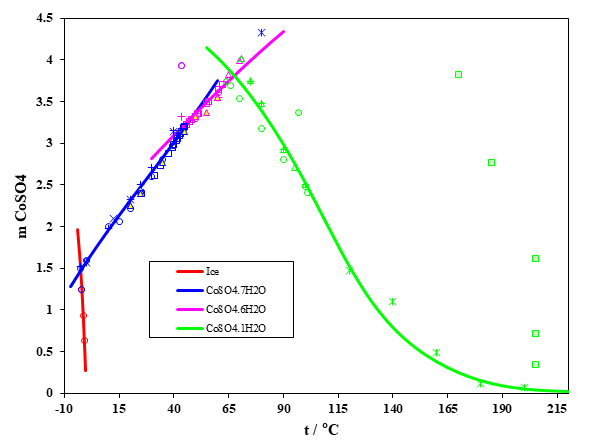
Figure 2. CoSO4 solubility in water as a function of temperature
Figure 3 shows that the OLI MSE model reliably predicts the solid-liquid phase equilibrium in the ternary system MnSO4-H2SO4-H2O. From 12.6 °C to 96 °C, the model accurately predicts five solid phases including three different double salts as well as the transitions between hydrates, hydrate-acid sulphate, and acid sulphate-higher acid sulphate, and two hydrates which are MnSO4.5H2O at low temperature and MnSO4.1H2O at high temperature. Dehydration of normal sulfate hydrates occurs as the temperature is raised and acid sulphates are formed as the acid concentration is increased.
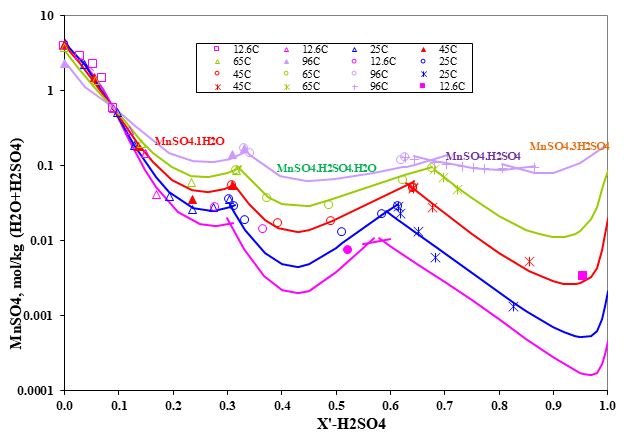
Figure 3. Ternary system MnSO4 – H2SO4 – H2O
Figure 4 shows that the OLI’s MSE model reliably predicts the ternary system Li2SO4-K2SO4-H2O. Formation of double salt between Li2SO4 and K2SO4 significantly affects the recovery process for lithium from saline brine. Therefore, the solubility diagrams of such similar systems are particularly important for this technology. The double salt Li2SO4. K2SO4 becomes congruently soluble at above 35 °C and its existence field broadens with increasing temperature. The results from the MSE model provide insights and a theoretical foundation for the separation of lithium and for studying its geochemical behavior.
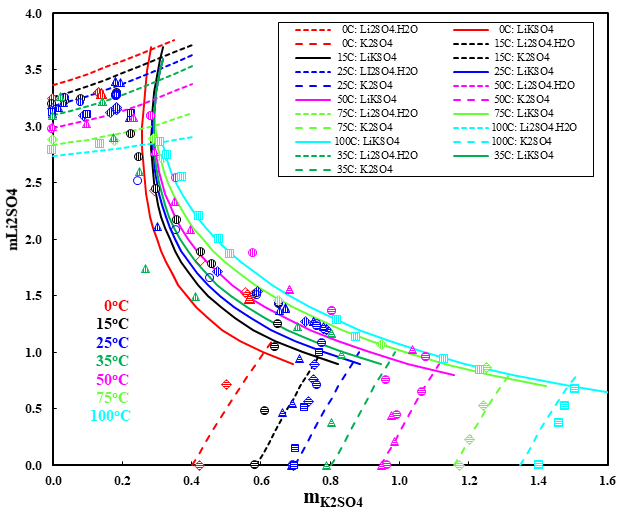
Figure 4. Ternary system Li2SO4-K2SO4-H2O
Through sulfuric acid leaching, the cathode material of spent LIBs is transferred to the products of Li2CO3, NiSO4, CoSO4, and MnSO4, all of which have higher added values. It is important to understand the selected transition metal sulfates in hydrometallurgical recycling of lithium batteries. As an example, Figure 5 shows the ternary system NiSO4-MnSO4-H2O. The prediction matches the experimental data very well. Four different hydrates precipitate at different temperatures and concentration of solutes.
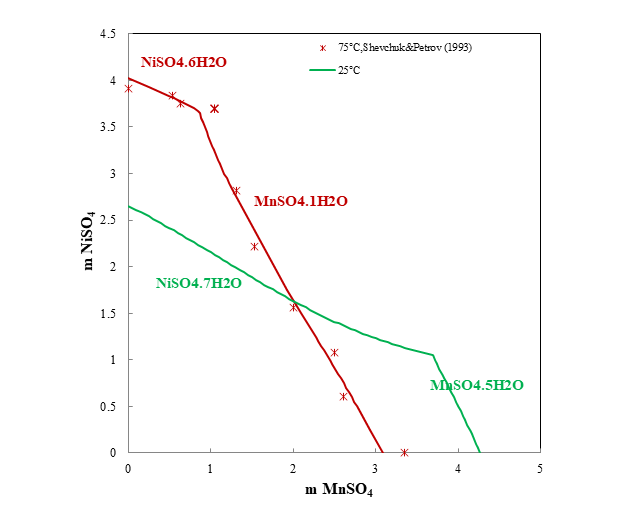
Figure 5. Ternary system NiSO4-MnSO4-H2O
What tools are available for LIBs recycling process?
The OLI MSE framework is uniquely positioned to model this process, and MSE is a proven model that can accurately simulate complex, multi-component electrolyte systems. It provides engineers with the ability to design more efficient processes, and to predict more closely than ever before the yield and purity of their products.
OLI Systems’ simulation packages, which is based on the MSE model, is available in OLI Studio V11.5 and OLI Flowsheet ESP V11.5.
Contact OLI at https:/www.olisystems.com/contact for more information or to schedule a meeting with an OLI expert.
References
- Arshad, F., Li, L., Amin, K., Fan, E.S., Manurkar, N., Ahmad, A., Yang, J.B., Wu, F., Chen, R.J., 2020. A comprehensive review of the advancement in recycling the anode and electrolyte from spent lithium-ion batteries. ACS Sustain. Chem. 8, 13527–13554. https://doi.org/10.1021/acssuschemeng.0c04940.
- Wang P., Anderko A., Young R. D., “A Speciation – Based Model for Mixed – Solvent Electrolyte Systems”, Fluid Phase Equilibria, 203, (1-2), 141-176, 2002.

