RESOURCES | post
Author: DaveS
Carbon Capture, Transportation, Utilization, and Storage (CCTUS) is expected to be pivotal in reducing CO2 emissions and combating global warming. The capture process may introduce several impurities into CO2, including nitrogen dioxide (NO2), sulfur dioxide (SO2), oxygen (O2), hydrogen sulfide (H2S), and water (H2O), which could appear at parts per million (ppm) levels. The specific mix of these impurities depends on the source of the flue gas, the CO2 capture technique employed, and various other factors. These impurities, due to their reactive nature, can lead to the formation of an acid-rich phase containing nitric acid (HNO3), sulfuric acid (H2SO4), or both. This poses a risk of corrosion to carbon steel pipelines, jeopardizing the safe transport and injection of CO2.
The injection of CO2 for storage demands tubing materials that can endure the corrosive conditions determined by near-wellbore factors such as pressure, temperature, brine salinity, pH, and the composition of injected CO2, among others. Once more, the presence of impurities like acid gases such as sulfur oxides (SOx) and nitrogen oxides (NOx), and their reactivity and tendency to form strong acids, can lower the pH and enhance corrosivity [1]. At the bottom hole during injection, formation water can become saturated with dissolved CO2. The introduction of dried supercritical CO2 can extract water from the brine, leading to increased salinity in the surrounding brine. In extreme cases, this may result in salt precipitation, exceeding solubility limits. Additionally, impurities will distribute between the water and vapor/dense phases with the acidic gases in CO2 reacting with formation water and producing strong acids. There is significant corrosion risk at the meeting point between connate water and high-pressure CO2, and these two phases co-exist at the wellhead. Impurities amplify the risk during shut-ins and even after CO2 reinjection. For instance, H2S can initiate sulfide stress cracking (SSC), O2 can heighten localized corrosion, and the formation of acids will lead to high general corrosion rates [2]. Thus, predicting corrosion behavior in these injection environments is essential for making informed material selections and maintaining asset integrity.
OLI Systems has developed a corrosion modeling tool capable of predicting both the conditions under which corrosive phases may form and estimating a corrosion rate based on the predicted chemistry. This tool enables, for the first time, the calculation of corrosion rates in systems where water is not the primary solvent. This is particularly relevant in CO2 transport and injection scenarios, where water is present as an impurity and not as a solvent.
When developing a corrosion modeling tool, it is essential to consider several factors that influence the corrosion of metallic materials. These factors encompass alloy metallurgy, solution chemistry (for example the presence of impurities in the CO2-rich phase such H2S, NOx, SOx, O2 content), and environmental variables like temperature, pressure, flow regime. Obtaining this degree of information solely from experimental data would be prohibitively expensive, highlighting the importance of a predictive corrosion model. A reliable predictive corrosion model must meet two prerequisites:
- Use a thermodynamic model that can accurately calculate the solution’s relevant properties, such as pH and viscosity, as well as the speciation and activities of electrochemically active species, their diffusion coefficients, and others.
- Use a first-principles-based electrochemical model that, when combined with the thermodynamic model, creates a powerful tool for predicting corrosion rates.
The New MSE Corrosion Model
OLI has developed and integrated these pre-requisites into the New MSE Corrosion Model. This model utilizes the Mixed Solvent Electrolyte (MSE) Model [3-5], which has been validated and employed for over 20 years to accurately predict chemical and phase equilibria in multicomponent mixtures. It also predicts solution properties such as pH and transport properties like viscosities and diffusivities, which then feed into the OLI corrosion model.
This new corrosion model includes partial anodic and cathodic reactions involved in the corrosion process and can model active corrosion, the transition from active to passive states, and the dissolution of the passive layer. To date, the model incorporates two duplex stainless-steel alloys: alloy 2507 and 2205. It predicts their corrosion rates in acid mixtures containing both oxidizing and non-oxidizing acids, and the effects of aggressive species like chlorides and fluorides, as well as inhibitive species such as nitrates.
The practical implications of the MSE corrosion model are vast, extending to the evaluation of material selection for CO2 transport and injection applications, showcasing its potential to significantly impact industry practices.
Description of the Electrochemical Model: The MSE Corrosion Model
The electrochemical module of the MSE Corrosion Model encapsulates the kinetics of electrochemical surface reactions for calculating corrosion potential and corrosion rate. This model simulates active dissolution, the transition from active to passive states, and passive dissolution. It is developed by integrating various partial anodic and cathodic reactions, utilizing the mixed potential theory. This theory states that the total anodic and cathodic currents are equal, represented as:
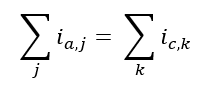
where and are the j-th anodic and k-th cathodic process, respectively. The model addresses:
- Alloy Dissolution in the Active State: Modeled through the Butler-Volmer equation, considering the adsorption of electrochemically active species. The surface coverage fractions of these species, correlated to their activities through an adsorption model, inform the dissolution process.
- Active-Passive Transition: Captured by incorporating a current representing the formation of a passive layer alongside the current for active dissolution. With appropriate parameterization, this formulation represents the critical current density, Flade potential, and passive dissolution range.
- Solution Chemistry’s Impact on Passivity: The effect of solution chemistry on passive dissolution and active-passive transition is modeled by considering surface reactions between the oxide film and solution species. Such reactions result in the formation of surface complexes, which may either enhance or inhibit dissolution.
- Cathodic Reactions: The various oxidizing species engaging in cathodic reactions on the metal surface are modeled. The Butler-Volmer equation represents the current density for each cathodic reaction, which is expressed using the surface coverage fractions and, hence, activities of various species.
- Mass Transfer Effects: These are crucial for understanding the dynamics between surface activities and bulk activities of species, quantified using mass transfer coefficients dependent on the flow regime.
Among the partial cathodic reactions, the key cathodic reactions in the acid systems include:
- Reduction of hydronium ions (H3O+), the primary cathodic reaction in acid corrosion. Reduction of water molecules, predominant in neutral and alkaline solutions.
- Reduction of neutral species acting as proton donors, such as CH3COOH, especially in concentrated solutions where direct reduction is significant.
- Reduction of oxidizing anions, like nitrates, to lower oxidation states.
While the mathematical formulation of the electrochemical kinetics expressions remains the same as in previous studies [6-7], the present electrochemical model incorporates two key revisions:
- Using the MSE thermodynamic model for calculating species activities, offering enhanced accuracy over older models.
- Revising the treatment of hydrogen ions to consider hydronium ions (H3O+) instead of bare hydrogen ions (H+), improving speciation prediction accuracy based on thermodynamic evidence.
The parameters in the expressions for current densities are determined based on experimental data on corrosion potential, corrosion rate, and polarization behavior. Generally, these parameters are regressed from data for individual acid systems, and subsequent calculations for mixed acid systems are purely predictive.
Validation of the MSE Corrosion Model: Predicting Corrosion Rates in Single Acids
The corrosion rate predictions for super duplex stainless steels in various acid environments were performed using the MSE corrosion model described earlier. As this model is semi-empirical, it requires some experimental corrosion data to accurately replicate the corrosion behavior of specific alloys. Below, Figure 1 illustrates the corrosion behavior of alloy 2507 in aqueous sulfuric acid solutions, ranging from very dilute to pure acid. The model is capable of quantitatively predicting corrosion rates as a function of both temperature and acid concentration. The data points represent experimental data gathered from literature, while the solid lines depict the predictions made by the MSE corrosion model. This system demonstrates that at low acid concentrations, corrosion rates increase. Then, at medium concentrations, the corrosion rates plateau, and as the concentration of sulfuric acid approaches high levels up to 100% pure acid, the corrosion rates significantly decrease. At very high concentrations (i.e., in an almost pure acid), the low availability of water leads to low protonation which causes the corrosion rate to drop. This system is a good example of how it is important to have accurate values of solution properties (such as the activity of hydronium ions) from the MSE thermodynamic model to be able to accurately predict the corrosion rate.
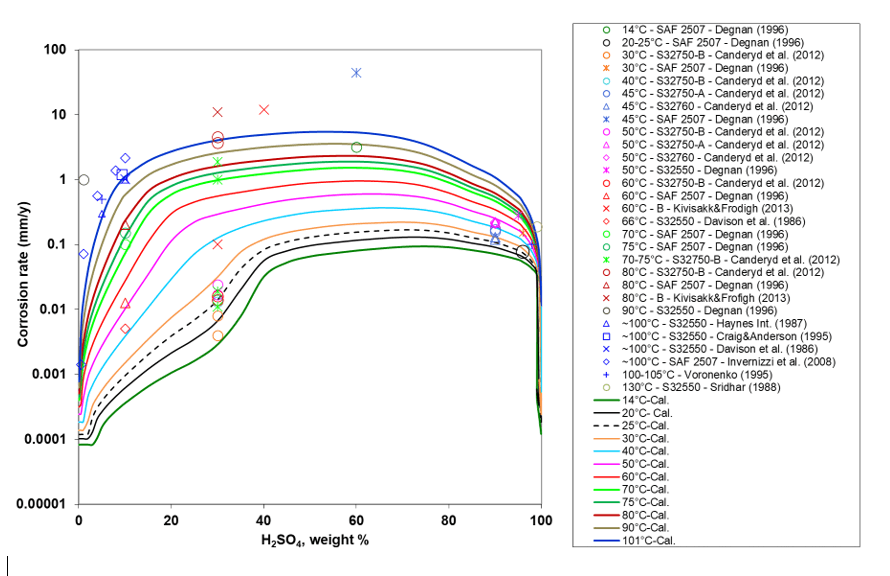
Figure 1. Corrosion rate of alloy 2507 varying with H2SO4 concentration for different temperatures. Symbols are experimental data and curves are model predictions.
To further validate the corrosion model, studies on alloy 2507 were conducted using HNO3. Figure 2 provides a comparison between experimental data points and the calculated corrosion rate data across a wide range of HNO3 concentrations and temperatures. Unlike corrosion in H2SO4, which is primarily governed by the acidity of the environment (quantified by the activity of hydronium ions), corrosion in HNO3 is influenced by the reduction of nitrate ions (NO3–) on the metal surface. Notably, as the concentration of HNO3 increases to higher levels, the corrosion rates decrease, similar to what was observed on the H2SO4 system. An important observation is that the corrosion rates in HNO3 are significantly lower – approximately two orders of magnitude – compared to those in H2SO4 under equivalent acid concentration and temperature conditions. It is important to emphasize that the corrosion model’s ability to predict the corrosion rates observed in experiments for a wide range of HNO3 concentrations, relies on accurate information about the NO3– ion activity, which is derived from the MSE thermodynamic model.
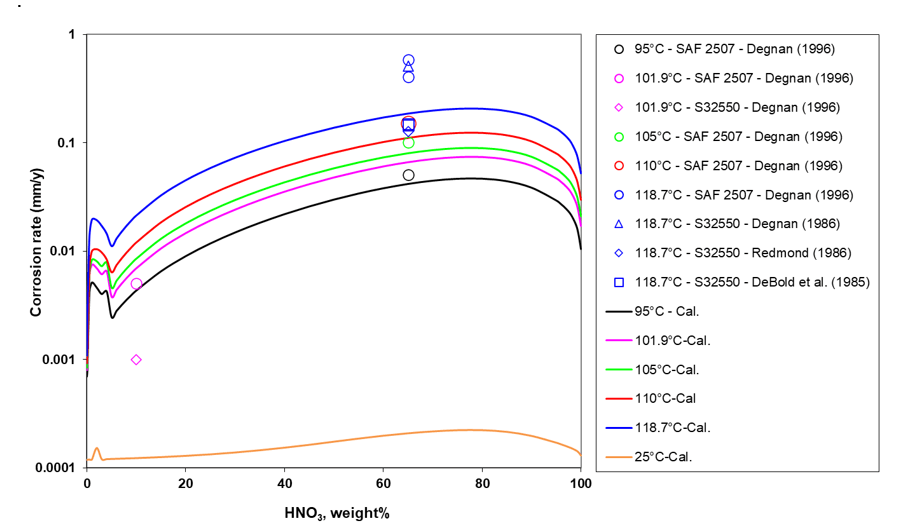
Figure 2. Corrosion rate of alloy 2507 varying with HNO3 concentration for different temperatures. Symbols are experimental data and curves are model predictions.
Validation of the MSE Corrosion Model: Predicting Corrosion Rates in Acid Mixtures
After demonstrating the model’s accuracy in predicting corrosion rates in single acids, it’s crucial to also highlight its predictive capabilities in acid mixtures. The corrosion behavior in mixtures can vary significantly, influenced by factors such as whether the acids are oxidizing or non-oxidizing, contain aggressive or inhibitive species, or are classified as weak or strong.
In scenarios involving CO2 transport and injection, the formation of acid mixtures, such as HNO3 and H2SO4, is plausible. Figure 3 illustrates the corrosion rates for alloy 255 in various ratios of sulfuric acid to nitric acid mixtures, showcasing the model’s ability to accurately replicate both temperature and concentration dependencies. The lowest line in the plot (turquoise line) represents a mixture of 15.6 wt% H2SO4 and 8.8 wt% HNO3, while the highest line (green line) indicates a mixture of 82.5 wt% H2SO4 and 5.8 wt% HNO3. Notice that both environments maintain similar HNO3 concentrations but differ significantly in H2SO4 concentrations. The increased presence of H2SO4 lowers the pH, enhancing the dissolution rate of the passive oxide layer and significantly elevating the corrosion rate. Therefore, at a given temperature, the corrosion rates can vary by nearly two orders of magnitude, depending on the acidity of the mixture.
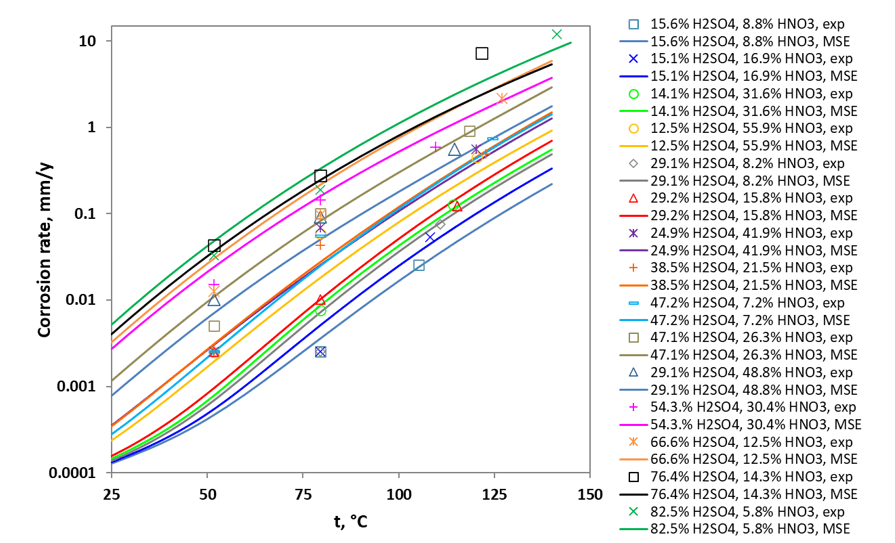
Figure 3. Corrosion rates for alloy 255 in H2SO4 + HNO3 solutions. The symbols are experimental data and curves are model prediction.
Concluding Remarks
The results presented demonstrate the MSE corrosion model’s ability to accurately predict general corrosion rates for both single acids and their mixtures.
The MSE thermodynamic model, designed to extensively model the chemistry of corrosive environments in multicomponent, multi-phase systems, has been integrated with a mixed-potential electrochemical model. This integration has led to the creation of the new MSE corrosion model, the first of its kind. This corrosion model aids in evaluating corrosive conditions and influences decision-making regarding material selection for CO2 transportation and injection applications.
REFERENCES
- H. Morland, A. Tadesse, G. Svenningsen, R. D. Springer, and A. Anderko, “Nitric and Sulfuric Acid Solubility in Dense Phase CO2“, Industrial & Engineering Chemistry Research 58, 51 (2019), p. 22924-22933.
- Material selection for anthropogenic CO2 injections: mechanical and corrosion performance of steels under dense CO2 with impurities. Cecile Millet, et al. Paper 18886. AMPP annual conference, March 2023
- Wang, A. Anderko, and R. D. Young, “A speciation-based model for mixed-solvent electrolyte systems“, Fluid Phase Equilib. 203, 1-2 (2002), p. 141-176.
- Anderko, Modeling of Aqueous Corrosion, in ‘Shreir’s Corrosion’, (ed. T. J. A. Richardson), Amsterdam, Elsevier; 2010), p. 1585-1629.
- Wang, A. Anderko, R. D. Springer, and R. D. Young, “Modeling phase equilibria and speciation in mixed-solvent electrolyte systems: II. Liquid-liquid equilibria and properties of associating electrolyte solutions“, J. Mol. Liq. 125, 1 (2006), p. 37-44.
- Anderko and R. D. Young, “Model for corrosion of carbon steel in lithium bromide absorption refrigeration systems“, Corrosion 56, 5 (2000), p. 543-555.
- Anderko, P. McKenzie, and R. D. Young, “Computation of rates of general corrosion using electrochemical and thermodynamic models“, Corrosion 57, 3 (2001), p. 202-213.
