Boron compounds have long played a crucial role in a variety of industries—from traditional applications such as glass and ceramics to high‐tech sectors like nuclear power generation and semiconductor manufacturing. OLI Systems and the University of Guelph joined forces to develop a comprehensive thermodynamic model for boric acid and alkali metal borate systems (P. Wang, A. Anderko, and P. Tremaine, Ind. Eng. Chem. Res., 62 (2023) 20875). This new model builds on earlier work by incorporating accurate experimental data from conductivity measurements, Raman spectroscopy, and solubility studies. The model now accurately describes both chemical speciation and phase equilibria over a wide range of temperatures (up to 623 K) and pressures. In this post, we explore the work’s key findings, discuss the most important reactions in borate chemistry, and examine the industrial implications of the model.
Challenges of Borate Thermodynamics
Traditional models of boric acid solutions assumed relatively simple behavior. However, boron chemistry is anything but simple. When boric acid (H₃BO₃) is dissolved in water, it undergoes a series of reactions that produce a variety of borate species. The new model refines our understanding by introducing a series of complex species – ranging from monoborate to pentaborate – based on accurate experimental results from the University of Guelph. Based on OLI’s Mixed-Solvent Electrolyte (MSE) framework, the model integrates state‐of‐the‐art Raman spectroscopic data and high‐temperature conductivity measurements to capture the nuanced behavior of borate systems under extreme conditions—conditions frequently encountered in industrial applications.
Key Reactions in Borate Chemistry
The complexity of borate chemistry results from the interplay of various chemical reactions including:
- Boric Acid Ionization:
In aqueous solution, boric acid reacts with water to form the tetrahydroxyborate ion (B(OH)₄⁻). This equilibrium is key to understanding the weak acid behavior of H₃BO₃. - Formation of Diborate Species:
Two boric acid molecules can condense—with the elimination of a water molecule—to produce the diborate ion (B₂(OH)₇⁻). This reaction becomes significant at higher boron concentrations and elevated pH. - Formation of Triborate and Higher Polyborates:
Further condensation reactions lead to the creation of triborate (B₃O₃(OH)₄⁻), tetraborate and pentaborate species. These sequential reactions are fundamental to the complex equilibrium observed in borate-rich environments. - Ion-Pair Formation:
Alkali metal cations (e.g., Li⁺, Na⁺, K⁺) interact with borate anions to form ion pairs (such as LiB(OH)₄, NaB(OH)₄, and KB(OH)₄). These associations significantly affect overall speciation—especially under the high-temperature conditions typical of reactor coolants. - Precipitation Reactions:
At elevated concentrations, borate species combine with metal cations to form solid phases. Predicting these precipitation reactions is essential for managing scaling in industrial systems, from nuclear power generation to geothermal plants to brine-processing operations.
The complexity of the chemistry manifests itself in complex phase behavior. The following two figures illustrate how the model predicts phase equilibria in borate systems.
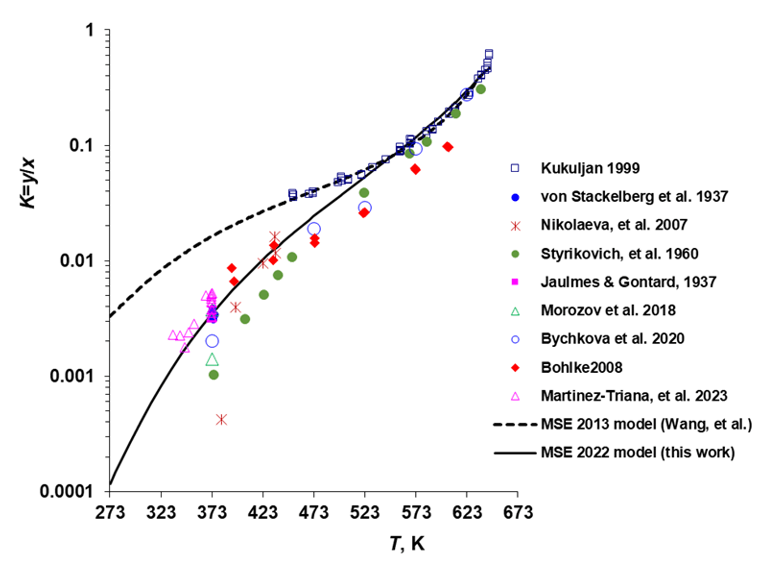
Figure 1: Volatility of Boric Acid as a Function of Temperature. The ratio of mole fractions of boric acid in the vapor and liquid phases is denoted by K=y/x.
Figure 1 displays the volatility of boric acid in the H₃BO₃ + H₂O system over a wide temperature range. The plot shows how the equilibrium vapor–liquid distribution of boric acid changes with temperature, providing insight into its vapor-phase behavior. This information is particularly important in applications such as nuclear reactor coolant management, where even minor changes in vapor composition can significantly impact safety and corrosion control.
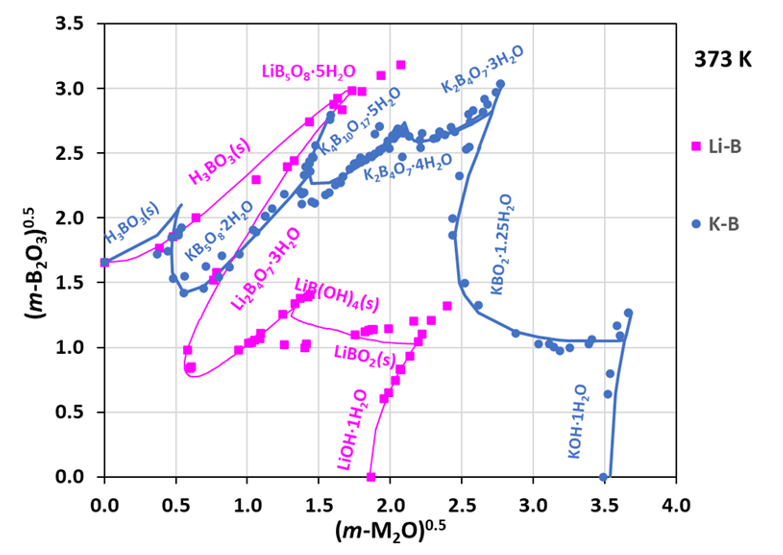
Figure 2: Comparison of solubilities in aqueous lithium borate and potassium borate systems at 373 K
Figure 2 compares the solubility behavior of lithium and potassium borate systems at a selected temperature (373 K). The pink curve represents the Li₂O + B₂O₃ + H₂O system, while the dark blue curve corresponds to the K₂O + B₂O₃ + H₂O system. In both cases, multiple phases can form depending on conditions, leading to a complex solubility diagram. Both experimental data points and model predictions are shown. Notably, the solubilities of lithium borates and their hydrates are significantly lower than those of the corresponding potassium borates, reflecting the distinct hydration and lattice energies of Li⁺ and K⁺ ions. This insight is important when considering alternative coolant chemistries for pressurized water reactors (PWRs).
Industrial Implications of the Borate Model
The borate model is not merely a theoretical advance—it has profound practical implications:
Nuclear Power and Reactor Coolant Chemistry
Boric acid is a standard additive in pressurized water reactors (PWRs), where it plays a crucial role in controlling reactivity. Accurate prediction of boric acid speciation under extreme conditions is vital for reactor safety. The model helps to:
- Predict pH and Reactivity: Improved speciation data enable better forecasts of coolant pH changes, which directly affect reactor control.
- Assess Corrosion and Crud Formation: Crud is the deposits that form on fuel cladding due to corrosion and impurity accumulation. Understanding the formation of solid borate precipitates and vapor-phase boron transport aids in mitigating corrosion and crud-induced power shifts (CIPS).
- Evaluate Alternative Coolant Chemistries: With an extended capability to model potassium borate systems, the model supports the evaluation of KOH as an alternative to LiOH, an important consideration amid lithium supply challenges.
Geothermal Energy and Brine Processing
Geothermal fluids and salt-lake brines often have high boron concentrations. The model facilitates:
- Scaling Control: Predicting conditions under which borate solids precipitate helps in designing strategies to prevent scaling in heat exchangers.
- Resource Recovery: Accurate phase equilibria data optimize the extraction processes for valuable elements such as boron and lithium from complex brine mixtures.
Materials Processing and Corrosion Management
Industries such as glass, ceramic, and semiconductor manufacturing benefit from precise control over boron chemistry. By accurately predicting borate solubilities and speciation, manufacturers can improve process control and minimize corrosion in production equipment.
Concluding Thoughts
The borate thermodynamic model marks a significant advancement in our understanding of borate chemistry. By integrating new experimental data and refining the speciation framework, the model delivers reliable predictions for both chemical and phase equilibria in systems ranging from simple aqueous boric acid solutions to the complex mixtures encountered in nuclear reactor coolants and geothermal brines.
In practical terms, these advances translate to:
- Enhanced safety and efficiency in nuclear reactor operations,
- Improved scaling management and resource recovery in geothermal and brine processing,
- Better process design and corrosion control in various high-tech and traditional industries.
The model described here is available in OLI software V12. For more information, or to consult and share ideas, feel free to contact us
References
- P. Wang, A. Anderko, and P. Tremaine, “Speciation and phase equilibria of aqueous boric acid and alkali metal borates from ambient to hydrothermal conditions: a comprehensive thermodynamic model,” Ind. Eng. Chem. Res., 62 (2023) 20875–20898.
- Wang, P.; Kosinski, J. J.; Lencka, M. M.; Anderko, A.; Springer, R. D.
Thermodynamic Modeling of Boric Acid and Selected Metal Borate Systems. Pure Appl. Chem. 2013, 85, 2117–2144. - Applegarth, L. M. S. G. A.; Pye, C. C.; Cox, J. S.; Tremaine, P. R.
Raman Spectroscopic and Ab Initio Investigation of Aqueous Boric Acid, Borate, and Polyborate Speciation. Ind. Eng. Chem. Res. 2017. - Arcis, H.; Ferguson, J. P.; Zimmerman, G. H.; Tremaine, P. R.
The Limiting Conductivity of the Borate Ion and Its Ion-Pair Formation Constants with Sodium and Potassium under Hydrothermal Conditions. Phys. Chem. Chem. Phys. 2016. - Sasidharanpillai, S.; Arcis, H.; Trevani, L.; Tremaine, P. R.
Triborate Formation Constants and Polyborate Speciation under Hydrothermal Conditions by Raman Spectroscopy. J. Phys. Chem. B 2019. - Ferguson, J. P.; Arcis, H.; Zimmerman, G. H.; Tremaine, P. R.
Ion-Pair Formation Constants of Lithium Borate and Lithium Hydroxide under Pressurized Water Nuclear Reactor Coolant Conditions. Ind. Eng. Chem. Res. 2017. - Arcis, H.; Ferguson, J. P.; Applegarth, L. M. S. G. A.; Zimmerman, G. H.; Tremaine, P. R.
Ionization of Boric Acid in Water from 298 to 623 K by AC Conductivity and Raman Spectroscopy. J. Chem. Thermodyn. 2017.