RESOURCES | post
Author: lisa.w
In 2020, global energy-related CO2 emissions reached 33 gigatons, contributing significantly to climate change, according to the International Energy Agency (IEA). The technology of using amines for carbon capture, known as “Amine-based Carbon Capture” is widely employed in industrial processes. Piperazine (PIP), a heterocyclic diamine, is notable for its rapid reactivity with CO2 to form stable and soluble compounds known as carbamates or bicarbamates, making it crucial for CO2 capture applications. Another key compound, methyldiethanolamine (MDEA), is extensively used to capture CO2 in power plants and industrial facilities. A significant advancement involves activated amine solutions, combining traditional amines like MDEA with accelerators such as piperazine, enhancing CO2 absorption rates in carbonate and tertiary amine solutions. H2S, as a gas impurity, might interfere with amine-based CO2 capture processes, thus capturing H2S along with CO2 can enhance the process efficiency, prevent corrosion, and ensure that these harmful emissions are not released into the atmosphere. Piperazine has the ability to selectively absorb CO2/H2S from mixed gas streams, making it a promising candidate for carbon capture processes. Predicting vapor-liquid, solid-liquid, and liquid-liquid equilibria and speciation in amine-based carbon capture methods is challenging due to the complex CO2-H2S-amine reactions and non-ideal behavior in real-world systems. High pressures and temperatures in industrial processes further complicate such predictions. The existing version of the OLI software (V11.5) offers support for several amines commonly used in CO2 capture processes (e.g., MEA, DEA, MDEA, DGA, etc.), but it does not include piperazine in its databank. This omission limits the utility of version 11.5 in CO2 capture applications. However, it is worth noting that piperazine will be incorporated into the upcoming OLI Software V12. Consequently, this article will concentrate on examining the significance of piperazine in CO2 capture. The application of version 12, featuring its speciation-based mixed-solvent electrolyte (MSE) thermodynamic model [1-3], greatly enhances the precision of simulations for CO2 capture under demanding conditions.
What makes OLI software stand out for modeling CO2 capture chemistry?
Carbon capture stands out as one of the five key sustainability areas that OLI Systems has prioritized its focus on. OLI as a pioneer in electrolyte and water chemistry modeling maintains extensive databases of experimental data for over 6000 species (more than 80 elements of the periodic table), which can be used to validate model predictions for CO2 capture as well as H2S absorption. These databases are continually updated and expanded, providing users with a valuable resource for model validation. OLI’s MSE framework can accurately forecast phase equilibria and speciation in processes that involve CO2 and H2S across a broad spectrum of operating conditions. This also includes the supercritical conditions, where the formation of a second liquid phase becomes thermodynamically feasible. This is particularly important for simulating amine scrubbing processes at high pressures. Knowing the partial pressure of CO2 during CO2 capture is crucial because the solubility and absorption of CO2 in the amine solvents and the overall CO2 capture efficiency are highly dependent on the partial pressure of CO2. Additionally, as the required energy for CO2 desorption step depends on the partial pressure of CO2, therefore being able to predict the CO2 pressure allows engineers to design and optimize their systems to maintain the balance between process efficiency and energy consumption. CO2 solubility is often characterized in terms of loading (moles of CO2 per mole of amines), indicating the amount of CO2 that a solvent can absorb [5]. Figure 1 illustrates a few examples of predicted CO2 or H2S partial pressures using the MSE model, which include the possibility of formation of two liquids under supercritical conditions. These predictions account for speciation calculations within aqueous piperazine/MDEA systems, showing strong alignment with benchmarked data. The model accurately captures the trends observed at both low and high loadings, particularly the significant drop in partial pressure of CO2 at low loadings, as demonstrated by the predictions. The strong drop of partial pressure of CO2 at lower amine concentrations demonstrates the efficiency of amines in capturing CO2.
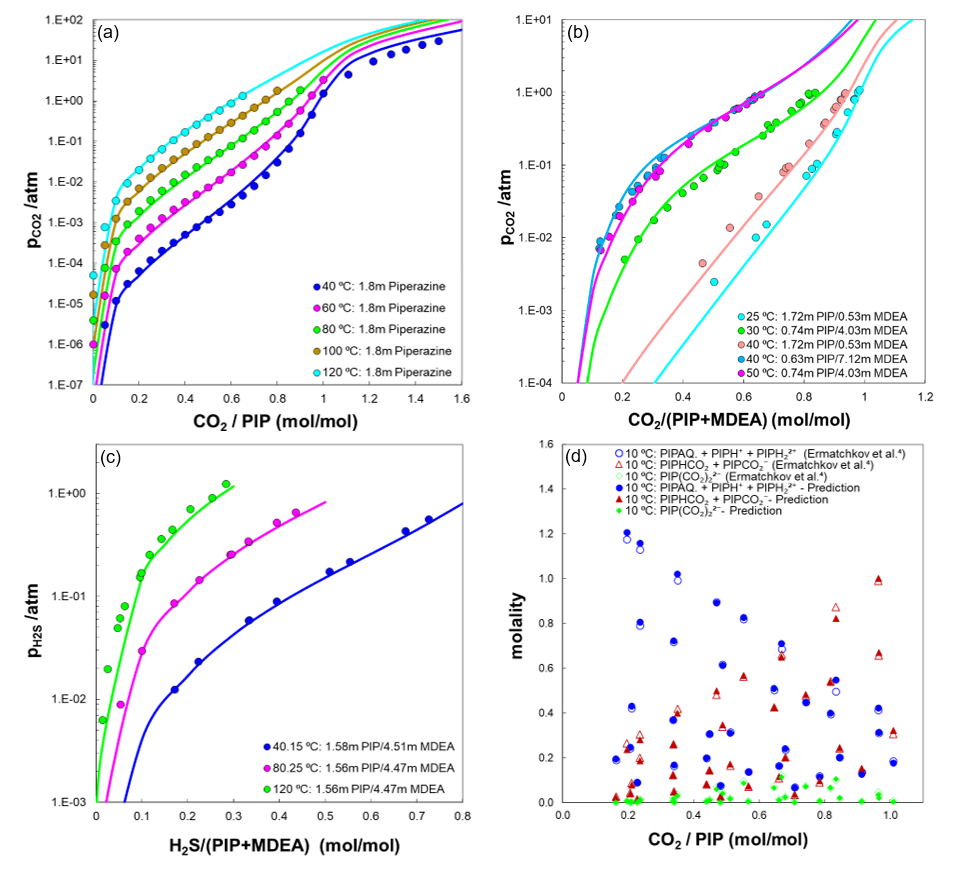
Figure 1. Predictions of (a) pCO2 in 1.8 m piperazine, (b) pCO2 in aqueous solution of PIP+MDEA, (c) pH2S in aqueous solution of PIP+MDEA, and (d) speciation in 0.1 – 1.5 m piperazine solution
Figure 2 shows the parity plot that compares the calculated and the experimental partial pressure of CO2 showing the solubility of CO2 in aqueous piperazine over a wide range of temperatures and piperazine concentrations. In the parity plot, the predicted values closely mirror the experimental data. This underscores the close alignment between the predicted and observed outcomes, encompassing a wide range of conditions and scenarios.
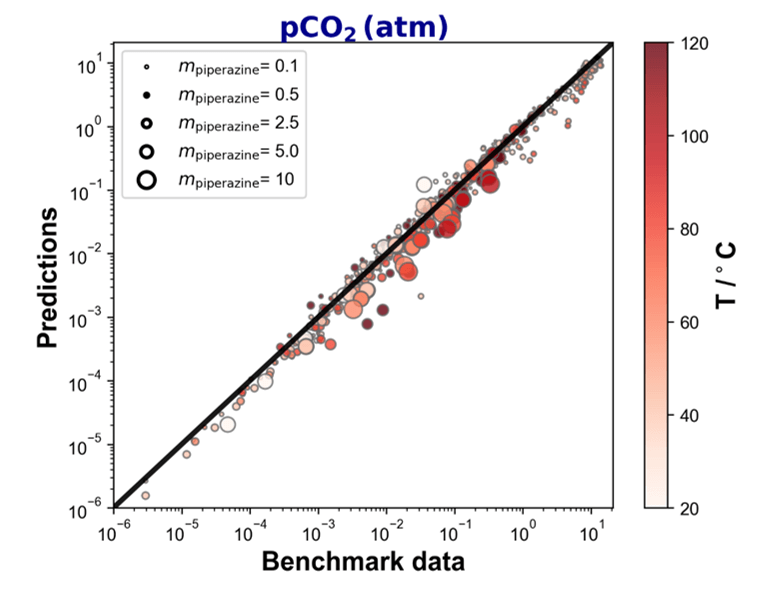
Figure 2. Parity plot of partial pressure of CO2 in aqueous piperazine
In summary, OLI MSE framework is specifically designed to accurately model the process chemistry, and it has a well-established reputation for accurately simulating intricate, multi-component electrolyte systems. It allows engineers and researchers to understand how fluctuations in feed composition, flow rates, and other factors can impact the process efficiency during amine-based CO2 capture or H2S removal. Furthermore, OLI Cloud APIs facilitate the incorporation of modeling outcomes into different simulation and optimization tools, enhancing the overall approach to the design and analysis of CO2 capture processes with a more comprehensive perspective.
To obtain comprehensive details about OLI’s chemistry solutions for enhancing carbon capture performance, please don’t hesitate to contact us.
References
- Wang, P., Anderko, A., & Young, R. D. A speciation-based model for mixed-solvent electrolyte systems. Fluid Phase Equilibria 203 (2002) 141–176
- Lencka, M. M., Kosinski, J. J., Wang, P., & Anderko, A. Thermodynamic modeling of aqueous systems containing amines and amine hydrochlorides: Application to methylamine, morpholine, and morpholine derivatives. Fluid Phase Equilibria 418 (2016) 160-174
- Jayaweera, I., Jayaweera, P., Kundu, p., Anderko, A., Thomsen, K., Valenti, G., Bonalumi, D., & Lillia, S. Results from process modeling of the mixed-salt technology for CO2 capture from post-combustion-related applications. Energy Procedia 114 (2017) 771–780
- Ermatchkov, V., Kamps, A.P.-S., & Maurer, G. Chemical equilibrium constants for the formation of carbamates in (carbon dioxide+piperazine+water) from H-NMR-spectroscopy. The Journal of Chemical Thermodynamics 35 (2003) 1277-1289
- Ermatchkov, V., Kamps, A.P.-S., Speyer, D., & Maurer, G. Solubility of carbon dioxide in aqueous solutions of piperazine in the low gas loading region. Journal of Chemical & Engineering Data 51 (2006) 1788-1796